well, heating them will distinguish
in general , how can carbonate vs. bicarbonate detection test be carried out??
-
UP 0 DOWN 0 0 7

7 Answers
another quesn....
Colourless salt (A) ->(heating in presence of NaOH) -> (B) (gas )
A -> CaCl2 soln. -> white ppt. (C)
(B) gives white fumes with HCl
(C) decolourises acidified KMnO4
What is (A)? Explain reacns?
another quesn...
A colourless salt (X) has 50% Na2SO3 and 50% H2O . What is the formula of (X) ? How much of SO2 at NTP is obtained when 2.52 g of (X) reacts with excess of dil.H2SO4?
Q. A colourless salt (X) has 50% Na2SO3 and 50% H2O . What is the formula of (X) ? How much of SO2 at NTP is obtained when 2.52 g of (X) reacts with excess of dil.H2SO4?
Ans:
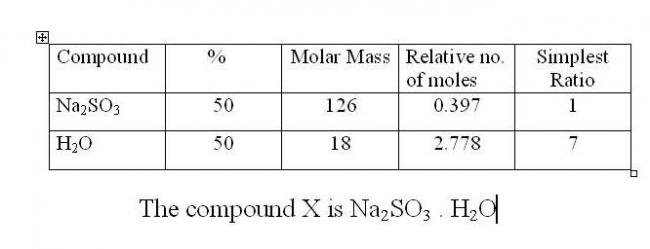
Molecular Mass = 126 + (7 x 18) = 252
2nd part :
Na2SO3.7H2O → Na2SO3 + 7H2O
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
Now applying unitary method,
252gm Na2SO3 gives 64gm SO2 ..
So, 2.52gm Na2SO3 gives 0.64gm SO2..
0.64 gm of SO2 will be formed..
I think The anser to ur previous question is Ammonium Chloride.
When Ammonium salts r heated in presence of NaOH pungent smelling gas NH3 is released.
When a glass rod dipped in HCl is brought near da mouth of the test the white ammonia fumes intensify.
Wen CaCl2 is added to salt A the acid radical in the salt A is replaced by Cl.
hence the white ppt. is of NH4Cl.
AND chloride salts decolourise acidified KMno4.