What I meant sir,was that if aluminium chloride generally existed in dimer state, then why would it react at all, if no altered form of it is used (as in case of most reactions) ?
Aluminium Chloride (AlCl3) exists as a dimer in solid phase , named Dialuminium Hexachloride (Al2Cl6) , with its octet fully filled . So it should have a good deal stability . But it still acts as a Lewis acid . Why is so ?
-
UP 0 DOWN 0 0 4

4 Answers
I don't think we use Al2Cl6 as lewis acid..we use AlCl3 as lewis acid
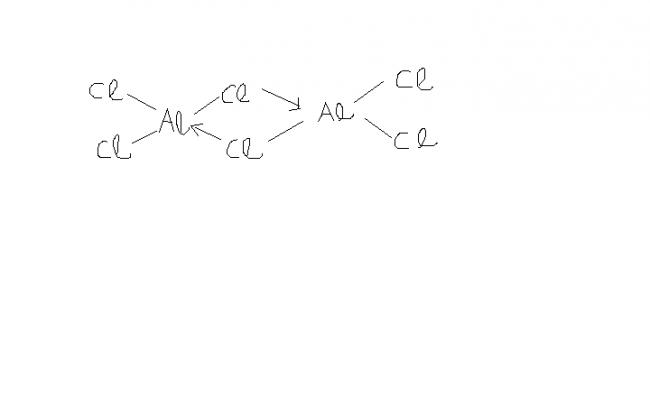
this compound hs complete octet, thus surely more stable thn its monomer state. also, its not a lewis acid.
soumya, many compounds hv der polymer state , but at a fixed state or temperature or condition , which may or mayn't be acheived easily.
same is the case here. this dimer of Al only exists in intermediate temperatures. i mean, not high ,not low temp. which cn not always be maintained. in ny other condition its dissosiates into its monomer.