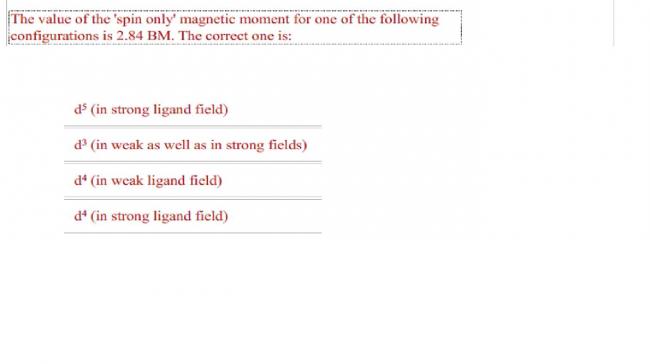
d4 strong field
7 Answers
Answer according to me is (D)
2.84 is √8 and so
By the formula n(n+2) u get the value of no of unpaired electrons as 2
And apart from the orbital diagram we need to concentrate on the crystal field theory.
According to that u hav two sets of orbiatls t2g and eg.
And when u insert an electron the e- enters the one with lower energy that is t2g . SInce the t2g has three orbitals the
in case of D u hav the first three e- going into the respective orbitals in the t2g sets and the third one joins in the first orbitaldue to strong ligand field
Hence there are 2 unpaired e- so the magnetic moment is 2.84 BM.
Tell me if anything u dint uderstand.
awesome explanation
but please tell y is this not possible in case of weak field ligand
because in the case of weak field ligand u hav the last e- going into the eg set which has higher energy and so the no of unpaired e- become 4 and magnetic moment becomes √24 which is about 4.92.