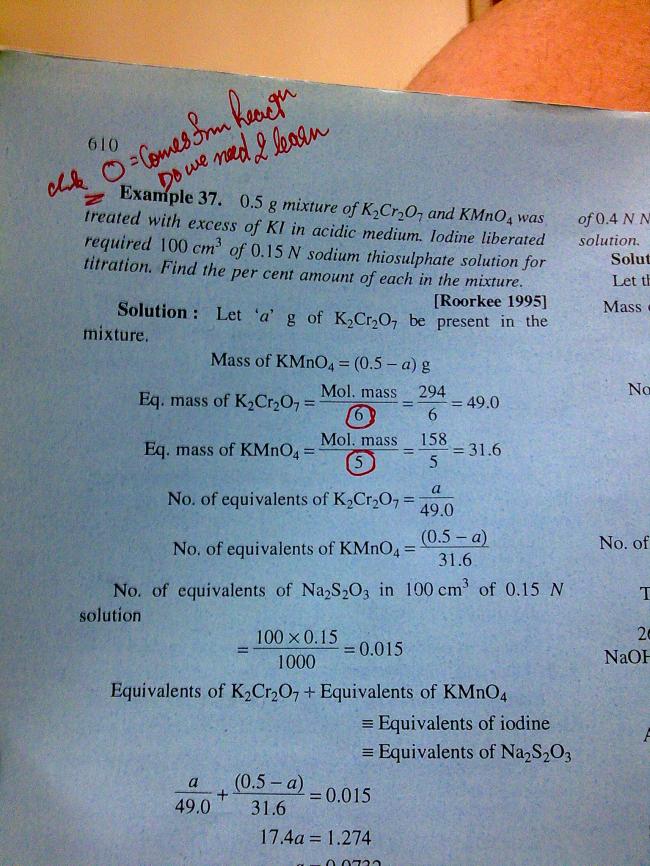11
11bhai dont get confused by the factors
write the reactions u will come to know about it .it represents the no of moles req for the oxidation /reduction of 1 mole of substance
(EDITED TO PREVENT CONFUSION)[1]
 21
21ARE U SURE : dat it is da valence charge charge present on Cr & Mn respectively? Mujhe to laga tha it wud b change in ox. state of Cr & Mn Pre and Post REACDTION wid KI in acidic med.
CAn U tell me y do u say its valence charge nad not the CHANGE IN OX. STATE
 21
21CHK AGAIN : CHARGE ON Mn in KMnO4 = 8-1 = 7
 11
11bhai read the 1st statement
REACTIONS R TO BE WRITTEN OFF TO GET THE SOLUTION
THE CHANGE IN THE OXIDATION NUMBER IS THE THING WHICH COUNTS...................
 1
1yes tapan u are right..
wahi hai jo tumhe laga tha..
it is the change in oxidation state..
not the valence charge
Mn 7+ -----> Mn2+ change of 5 .. so eq wt= mol wt/5
Cr2 12+ -------> Cr26+
change in ox state per formula wt = 6
so eq wt = molwt/6
i think it is ok now... [1]
 1
1yehi toh hain na...n-factor....for kmno4 it is 5 and for for cr it is 6
.: eqwt...= mol wt/ n-factor
hey...im rite na ...im not confusing any1?
 21
21Aarti : hope u know : n-Factor is not CONSTANT for a compound!
It depnds on the product wich helps find CHANGE IN OX. STATE!!!
 21
21YAH!!! absolutely!! Thnx [1]
 1
1yah ...i knoww...dats y i told dat for dis rxn...da n fctor for mn and cr dey are 5 and 6 respectively