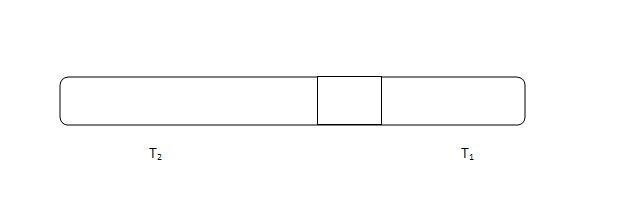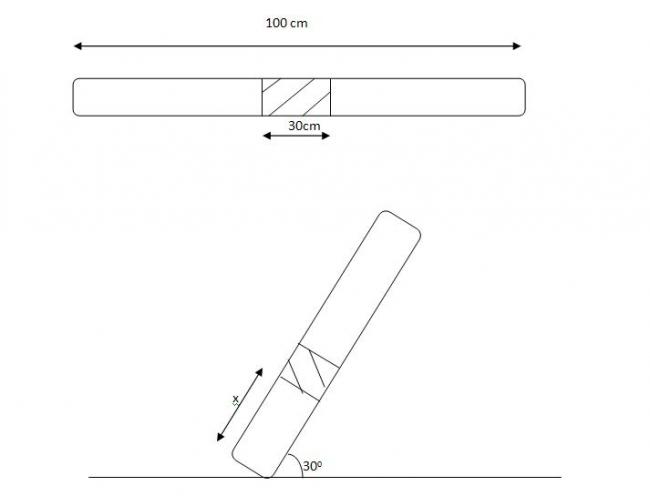
a glass tube with mercury trapped in b/w.
length of glass tube 100 cm lenthg of mercury in b/w glass tube of length 20 cm(on both side equal air columns at normal condns)
take density of mercury =Ï find :
a) ht of air column above when tube is kept 300 with horizontal
b) tube is now in horizontal condn one end is kept at 327 K other at 427 K find widths of air columns.....
-
UP 0 DOWN 0 0 8

8 Answers
Hey i think of this question..
but u make very good photoo.. what software u used?
b) 39.6cm and ( 70 -39.6) = 29.4cm
a) I think this approach helps.
Pressure in the lower part is equal to the pressure due to the mass of Hg and the pressure of the gas above.
Yes the approach is correct and if ur calculation is correct so will be the answer.
Yes consider FBD of Hg and balance the forces on Hg