My Contribution.......
Good work guyss
The halogen derivatives of aliphatic and aromatic hydrocarbons are called Haloalkanes and Haloarenes, respectively.
Haloalkanes are commonly known as Alkyl halides and Haloarenes as Aryl Halides.
There are two types of isomerism seen in halo compounds: chain isomerism and position isomerism.
Alkanes react with halogens in the presence of light to give Haloalkanes.
Addition of Halogen acids to alkenes also gives Haloalkanes. In case of unsymmetrical alkenes, Markovnikov's rule is applied.
Haloalkanes can also be prepared from alcohols by reaction with halogen acids, phosphorous halides and thionyl chloride.
Thionyl chloride method is the best since the byproducts are gases which easily escape.
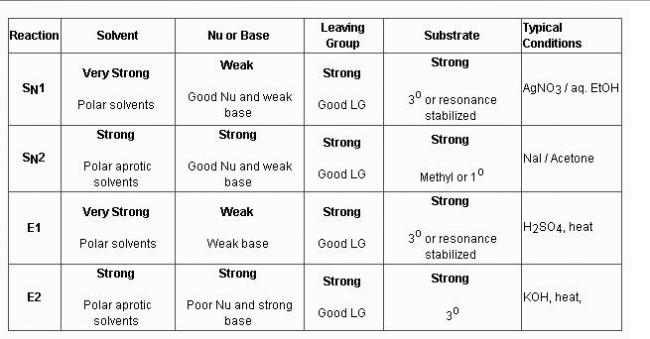
The polarity of the C-X bond of alkyl halides is responsible for their nucleophilic substitution, elimination and their reaction with metals.
Nucleophilic substitution reactions are categorized as unimolecular substitution and bimolecular substitution.
Unimolecular nucleophilic substitution reaction is favoured by tertiary alkyl halides while bimolecular nucleophilic substitution is favoured by primary alkyl halides.
Polyhalogen compounds are carbon compounds with more than one halogen atoms.
Most of them are very useful in industry and agriculture like chloroform, iodoform, freons and DDT.
Iodoform test is used to detect the presence of aldehydes and ketones that have at least one methyl group attached to the carbonyl group.
-
UP 0 DOWN 0 1 2