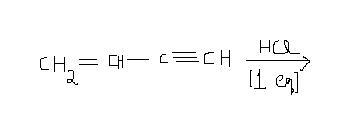double bond is attacked preferentially and the more stable carbocation is generated,,,chlorine gets attached there !
9 Answers
thats what i thot but aakash gave anser as the one with triple bpnd i.e. CH2=CH-C(Cl)=CH2 it said reason was allylic carbocation
but wont it also be vinylic?
further resonance leads to cumulated diene which is less stable than isolated dienes as well . so resonance is ruled out so should the answer be
CH3-CH(Cl)-C≡CH ??
the main idea here is that a double bond is more reactive towards electrophilic substitution that a triple bond !
answer given by akash is rite ....thsi is conjugated diene naa...whic his most stable
CH2=C-C=CH2
l l
H Cl
no way.. in electrophilic addition RDS is the formation of the carbocation.. (attack of electrophile) product has no relation with the rate
First of all, this is electrophilic addition, not substitution, minor issue but never confuse the two.
Secondly, the rate determining step of an electrophilic addition is indeed the formation of a carbocation, the more stable carbocation leads to the product. We also have to look at steric and electronic factors in the carbocation.
Suppose I land the proton on the triple bond, the carbocation so formed will be allylic. But when I resonate the carbocation, it will lead to a cumulated diene intermediate, which is less stable than an isolate diene intermediate, as Asish said. There is no scope for further stability by resonance here, and the product formed would be a non-resonance one, simple H-Cl addition across the triple bond.
However, if I were to follow a different pathway altogether, to avoid the obstruction of a cumulated diene and obtain a secondary carbocation in any case, I would land the proton on the double bond. There would be no resonance, but a secondary carbocation is stable enough. So I think preferentially the double bond would be attacked in this case.
If I can't resonate for stability, why even follow that pathway? Some of the cases where resonance leads to unstability...the product formed from this pathway will be a minor product if more than 1 eq of HCl is used.