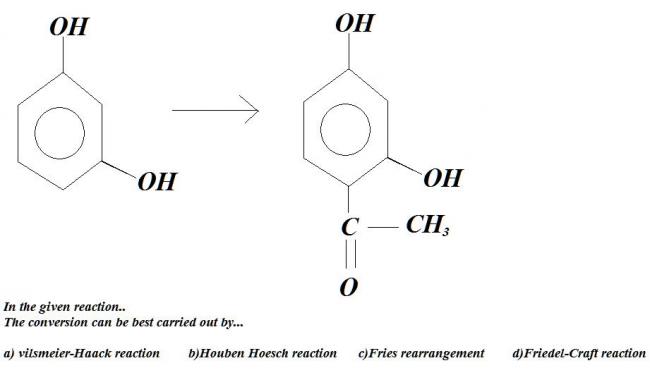
i thnk it's correct not sure ??
9 Answers
Friedel Craft's right.
Am saying this even though i don't remember hearing a word about the 1st 2 reactions :)
i think it's Houben Hoesch...
it's used to introduce acyl group in polyhydric phenols..
n why not friedel crafts.................neways rest three r not in iitjee
rickde that's not right. Fries rearrangement is there in IIT-JEE :).
Heat m-hydroxy phenol with one mol RCOOH. We get a m-hydroxy ester. Now react this with 1 mol Lewis acid, and we get our product straightaway by Fries rearrangement.
ok ok dinna see fries..
but still wat is the problem wit fridel crafts
@sandipan.....na na tat was my advice not to read strange rxn wit strange names...it wasn't my 'logic'
The Hoesch reaction or Houben-Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form a aryl ketone. the reaction is a type of Friedel-Crafts acylation with hydrochloric acid and a Lewis acid catalyst.

Source: Wiki
Didn't find Hoben Houesch reaction in GMP, though it seems the best way after reading what's given about it..