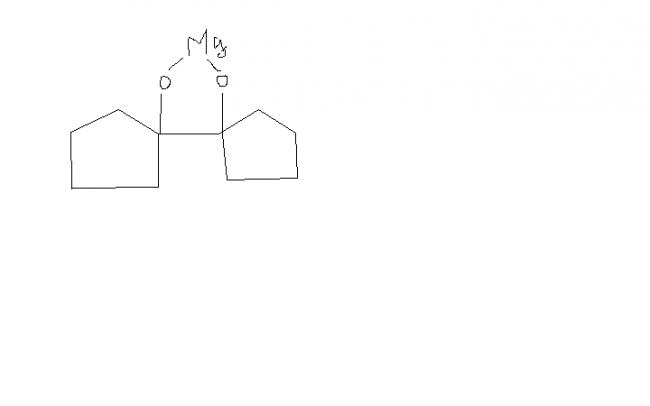
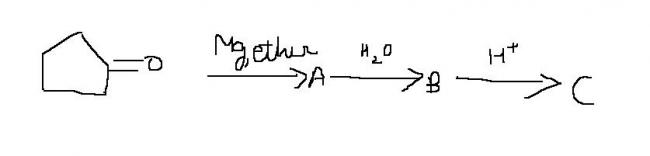
I tried this question. Can you post figure of C?? I'm not so sure of my steps ( and more certainly, I'm wrong this time).
So, plz. post the figure of C.
9 Answers
Pritish, can you explain your first step? Reaction of cyclohexanone with Mg/ether ? From where you are using MgBr.?
Ankur how did you get that dimer? Mechanism bata, what I thought of is just a fluke!
nooo..... wait... guys wait.. I didn't solve that time by mechanism and that thing is wrong..I'm reposting.. wait..
And pritish, in which sense you are claiming it to be fluke?
Here is mechanism: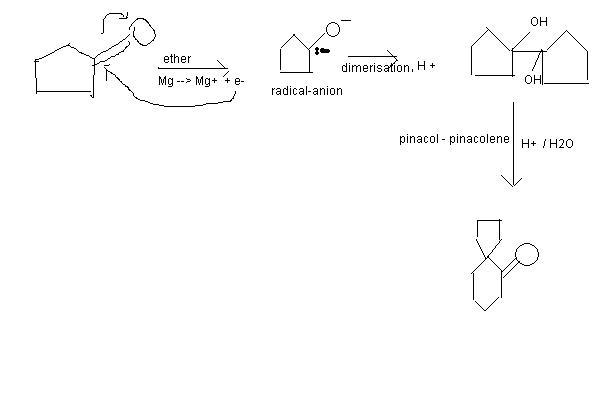 . The initial process is formation of pinacol and pinacol-pinacolene rearrangement proceeds.
. The initial process is formation of pinacol and pinacol-pinacolene rearrangement proceeds.
So it should be the answer.
Yeah, I'm not so good in radical-anion mechanisms. I initially thought Mg would donate electrons to the carbonyl bond, but didn't think of dimerisation. Yours is more logical.
ya, ankur. u r right. well done . just u hvn't mentioned A. doing it fr ya.