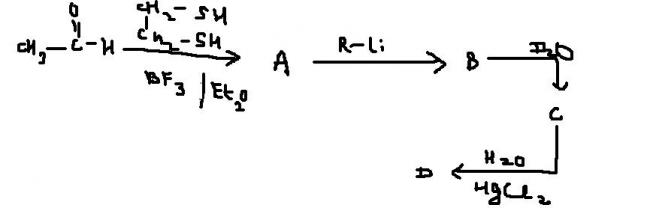but what abt BF3/Et2O ??
11 Answers
i think eure its sames as dat wat we do for ethylene glycol.(it protects the aldehyde group.)
yaa, as glycol adds to carbonyl compound to form dioxolane, similarly thioalcohol reacts to form mercaptal Difference b/w dioxolane and mercaptal:
Acetal is stable in the presence of alkali but are converted into the aldehyde by acid. Thus acetal formation may be used to protect the aldehyde group against oxidizing agents. In acid solution, aldehyde group can only be protected by mercaptal formation.
Hope it is clear.
PS: I also think that as cleavage of epoxide by Lithium Aluminum Hydrate (LaH) is considered as acid catalysed (not sure), then after the formation of dioxolane, reaction of dioxlane to reduce any ketonic group to alcohol, LaH will instead decompose dioxlane back to aldehyde.
what is this msp ? you are giving a wrong notion about your powers....what was so important in the post of yours in this thread that you have pinked it?? this has occurred in many other occasions also!! it is not at all right !
thanx ankur..that was good info..
and plz debo stop fighting over pinks....thats going to lead us nowhere....[1]
its just the knowledge and not pinks which help in JEE
wait wait.. I was mad.. silly me.. I didn't see, I told everything about mercaptal formation.. duh.. but you asked about another thing.. just see this, you will understand: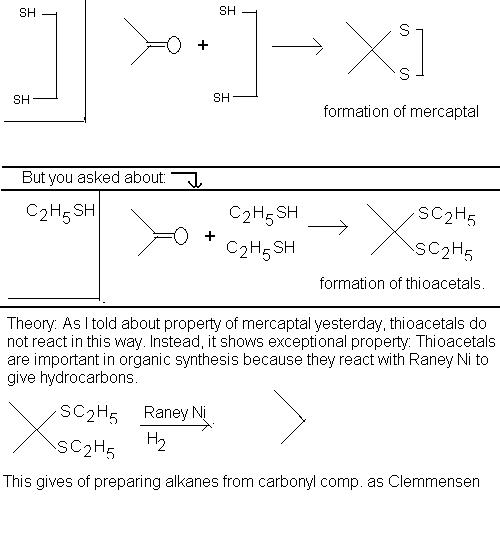 .
.
Hope I made it?
hey organic i din pinked that one,mind ur words.i have seen u in many places blaming me.If u want pinks go and ask the admins and fight with them,y are u fighting with me.You may be brilliant but don think that u are the most intelligent person.
i refrain from a fight here, but the fact is there was nothing in the post to get it pinked and attract the attention of the users !!!