The product which you can't find a mechanism for will obviously be a minor product...cyclohexanone is the major product.
See, first when the right side alcohol leaves as water, there is a ring rearrangement and pi bond formation to give cyclohexanone as major product.
The second product I can't think of...
The third product(your last image) is obtained by acidifying the left side alcohol. Now carbocation rearrangements occur irrespective of the fact that the cation is tertiary...it is just that the tertiary cation yields the major product in terms of proportion. A hydride shift from the right side -CH2 to the tertiary cation makes it primary. Hereon pi bond formation finishes the reaction, giving your (minor) product.
What are the 3 products obtained in the following reaction?
I got 2 but can't get the third one :(.
Answer is given -
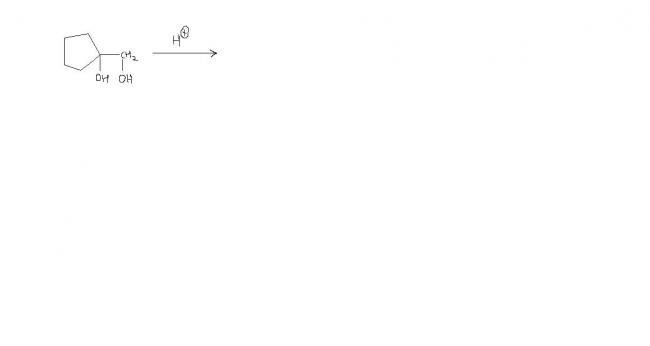

Can someone explain the mechanism for this product?
-
UP 0 DOWN 0 0 3

3 Answers
Pritish Chakraborty
·2010-11-22 19:59:42
qwerty
·2010-11-22 20:41:56
arey remember that
>C^{+}-OH\; \leftrightarrow\; \; >C=OH^{+}
as in second canonical octet is complete , this C+ is highly stable , even more stable than benzylic and allylic ....
so try to make some shifts to make that Carbon get the +ve charge wich is attached to an atom or a grp wich can show +M effect as Oxygen can