http://www.goiit.com/posts/list/organic-chemistry-which-is-more-acidic-formic-acid-or-benzoic-900544.htm
Bipin bhaiya's post.
8 Answers
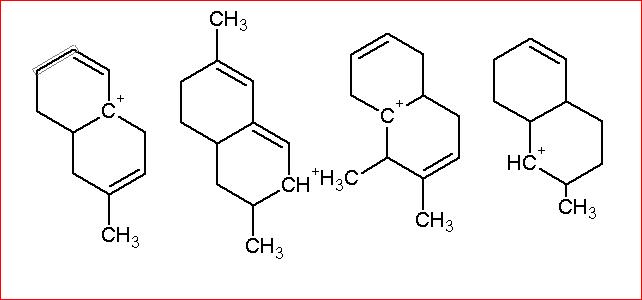
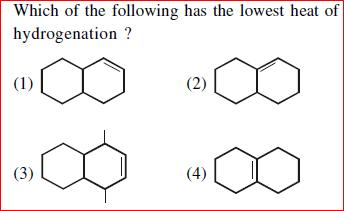
Arrange in order of increasing Stability.
In this case I tried to count hyp' structures but that won't give complete answer. I somehow managed to get the answer but need more explanation on role of methyl groups there and the double bonds in explaining the question.
My answer: 2>1>3>4
Resonance being a stronger effect in comparison to hyperconjugation, must get our first thought. The second structure has 3 R.S. whereas the first one has two, and the rest two do not have resonance because of the presence of intermediate sp3 carbon atoms. Clearly 2 is most stable, then 1. Now in 3 and 4. 3 has more hyperconjugative structures than 4 and hence the order. [1]
I will give another explaination to (1)
First of all think why is phenol less acidic than formic acid though phenoxide ion has more number of resonating structures than formate ion??
Reason is though phenoxide has more RS than formate, the participating RS in formate ion have equivalent content of energy unlike in phenoxide... that makes formate ion more stable and formic acid more acidic..
similarly, in benzoate tho the number of RS is more, formate is more stable because of equivalence in energy content in the RS of formate ion unlike in that of benzoate..
thus benzoate ion is less stable than formate ion..
thus formic acid is more acidic than benzoic acid! [1]