Q2. 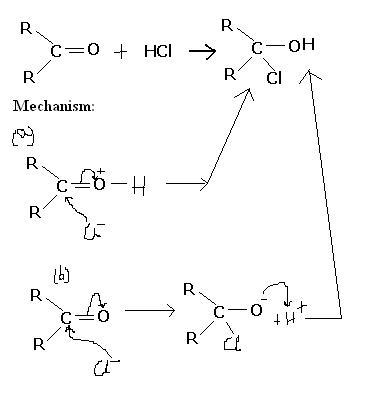
Both the reaction mechanisms explain the formation of the product. But if it was (a) then it wud have been electrophilic addition while in (b) it wud have been nucleophilic addition. But we know that it is (b).
WHY NOT (a) ?
Q1. Why is 1,4 addition product more stable than 1,2 addition product?
more substitued ALKENE is formed .... more no. of alpha H so more stable....
ii) Why is the 1,2-addition product (less stable) formed more rapidly at lower temperatures?
First, we need to look at the resonance stabilized allylic carbocation. We must consider the degree of substitution of both the positive carbon and the carbon-carbon double bond in each contributing structure.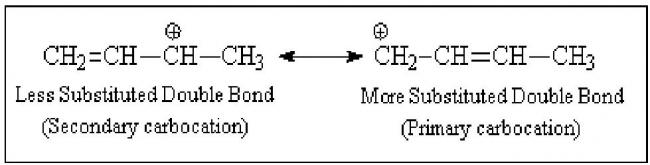
A secondary carbocation is more stable than a primary carbocation, and, if the degree of substitution of the carbon bearing the positive charge were the more important factor, then the Lewis structure on the left would make the greater contribution to the hybrid. A more substituted double bond is more stable than a less substituted one, and, if the degree of substitution of the carbon-carbon double bond were the more important factor, then the Lewis structure on the right would make the greater contribution to the hybrid.
We know from other experimental evidence that the location of the positive charge in the allylic carbocation is more important than the location of the double bond. Therefore in the hybrid, the greater fraction of the positive charge is on the secondary carbon. Reaction with bromide ion occurs more rapidly at this carbon, giving 1,2-addition, simply because it has a greater density of positive charge.
iii) Is the 1,2-addition product also formed more rapidly at higher temperatures, even though it is the 1,4-addition product that preodominates under these conditions?
Yes. The factors affecting the structure of a resonance stabilized allylic carbocation intermediate and the reaction of this intermediate with a nucleophile are not greatly affected by changes in temperature.
iv) Why is the 1,4-addition product the thermodynamically more stable product?
Generally, the greater degree of substitution of a carbon-carbon double bond, the greater the stability of the compound or ion containing it. Following are pairs of 1,2- and 1,4- addition products. In each case, the more stable alkene is the 1,4-addition product.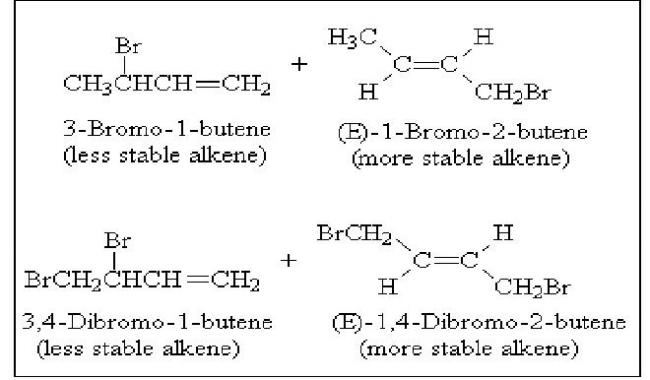
There are cases where the 1,2-addition product is more stable, and would be the product of thermodynamic control. Addition of Bromine to 1,4-dimethyl-1,3-cyclohexadiene under conditions of thermodynamic control gives 3,4-dibromo-1,4-dimethylcyclohexene, because its trisubstituted double bond is more stable than the disubstituted double bond of the 1,4-addition product.
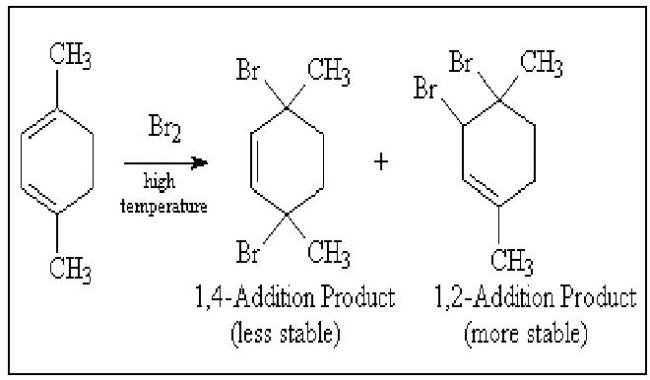
crditz------>copy pasted 4m somewhere
{thought could be useful
Q2. 
Both the reaction mechanisms explain the formation of the product. But if it was (a) then it wud have been electrophilic addition while in (b) it wud have been nucleophilic addition. But we know that it is (b).
WHY NOT (a) ?
my logic
in the presence of the aqueos medium
there will be H+ and Cl- in the medium
(a) mein asish tunne H ko pehle kaise atach kar diya?
(b) mein H+ react karefa in the last step
for more info
refer NCERT textbook
part 2 ka 1st chapter
uss mein clearly bataya hai
arey acid-base reaction. O mein δ- charge hai. So, it will add the H+