 1
1hey i got the mechanism for the first question !! [1]
jus now posting :)
 33
33yes... ur right...
as attack of lone pair of oxygen of per acid on carbonyl carbon is not that favoured in ketone..
so deouble bond..
 21
21and ppl. i read in an MTG book....
if there is jus 1 mol CF3CO3H it preferentially attacks da double bond not BVO!!!!
 1
1haan yes because the other two are resonance stabilised :)
 1
1haan ho sakta hai...
i jus anserewd by seeing the product...
 33
33in epoxy formation also O* is used..
 33
33But in mech only one oxygen is used..
wrong product given by him ....
or two moles of per acid was used..
 33
33its objective time...
just one point to mention in BVO..
CH3-C-O-O*-H
||
O
O* gets inserted... and O of ketone remain at its place so no need of mech now..
 1
1he gave that product re ....
 1
1if u want the mechanisms i can give...
but if u have, then its fine :)
 33
33wrong :(
one mole of peroxyacid does only BVO or epoxide formation not both by 1 molecule of peroxyacid..
one peroxyacid gives only one oxygen..
 1
1ITS BAEYER VILLIGER RCTN DUDE!!!!
 1
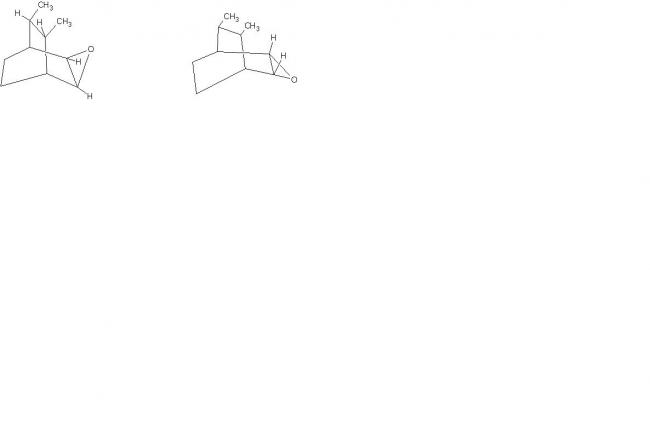
1here are your two diastereomers.
 24
24@ srinath, can u give the reason..............ur ans is correct..
 24
24some of u may have got it.......still i would be happy to know the mechanism.......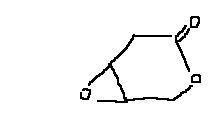
 1
1hahahahahhaha popat ho agay
neways org sucks
u post d answer guess we could make out d mch ;)
 24
24actually answer is very muich difff........
 1
1wats da answer to 1 eureka???
is it an epoxide or diol???
 3
3integrations can u explain me how u got the product
 3
3an epoxide will be formed for both the reaction
 1
1in bayer villiger we dnt hav a ene
