But r.d.s is the breaking of C - H bond
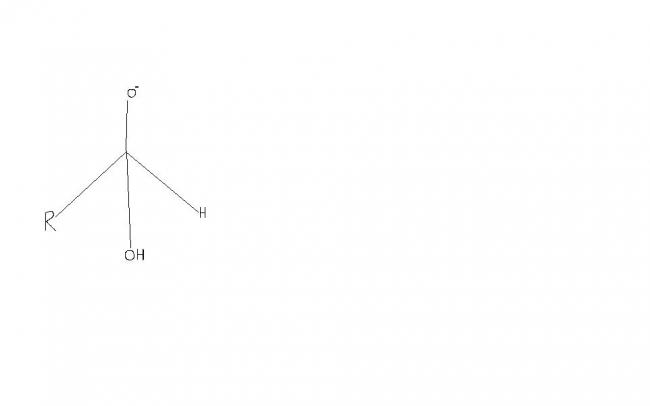
Give reason for the following
In Cannizaro reaction , presence of electron withdrawing group ('R') increases the rate of reaction
-
UP 0 DOWN 0 0 5

5 Answers
Lokesh Verma
·2009-01-16 02:37:44
I think it increases the stability of the intermediate formed on the attachement of OH-
Siddharth
·2009-01-16 04:03:48
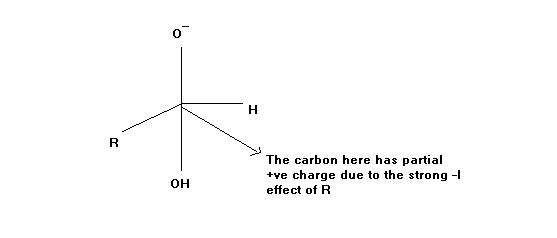
But R is an electron donating group.. It an alkyl or aryl group
And even in any case that R has a strong electron withdrawing group attached to it then -OH group will find it easy to attack that carbon(image)
voldy
·2009-01-16 06:02:56
it makes the strength of the C-O single bond more than that of the C-H bond .da
as O- can donate e-s better
Debotosh..
·2009-08-14 08:51:51
that makes the carbonyl carbon more electron deficient and hence expecting better attack from hydroxide ion !!!!