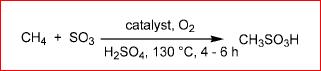
Why is it that alkanes lower than hexane cannot be sulphonated?
-
UP 0 DOWN 0 0 1

1 Answers
There could be several reasons and I cannot ascertain on a particular one.
Firstly, It is believed that the mechanism proceeds by a free radical one, So generating a Stable free radical for linear alkanes of one or more carbons (but less than 5 or 6) is difficult I consider.
Secondly, Sulfonic acids of lower alkanes are highly acidic (Consider methanesulfonic acid,its pKa = -1.9, is also about one million times stronger acid than acetic acid ) are hence are Volatile in nature. This greatly reduces the yield of the Product which is not desirable.
However, Under Suitable Conditions sufficient improvement has been achieved in the yields.