1. a)
2. a)
3. b) and not a).
1.Which of the following statements is true regarding nucleophilic substitution-
(a) Allyl chloride is more reactive than vinyl chloride .
(b)Vinyl chloride is as reactive as allyl chloride
(c)Vinyl chloride is more reactive than allyl chloride
(d)Both of them are reactive than chlorobenzene
2.The hydrogen atom in choloroform is
(a)acidic
(b)basic
(c)neutral
(d)none of these
3.Cholropicrin is -
(a)picric derivative
(b)nitrochloroform
(c)nitromethane
(d)nitroethyl chloride
-
UP 0 DOWN 0 1 7

7 Answers
Ans 1..A..in vinyl chloride there is a partial double bond b/w C-Cl
Ans 2..A ..it is acidic as the anion formed is stabilised by resonance
Ans3..CCl3NO2 is choloropicrin so B
Cl has empty d orbitals...though the resonance will not be that gud as there are 2p of carbon and 3d orbitals chlorine..but we cant say that resonance wont be there..and yes Inductive effect will also stabalise it..
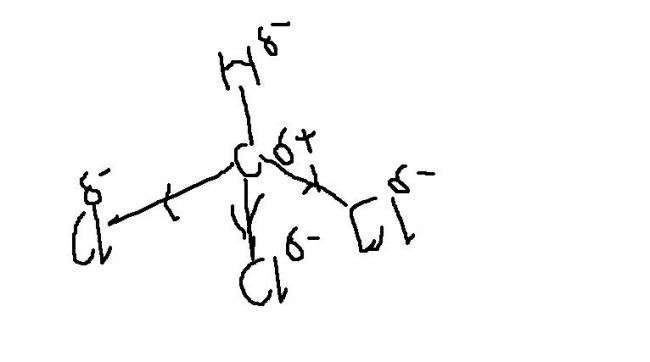
As the three chlorine atoms are electronegative ,they acquire a negative,due to which there is partial positive chage on C due to whcih there is partial negative charge on H hence H- will be given out instead of H+......so how is it acidic??????
well to both pritish n nix_13
after removal of H+ the anion thus resulting is stabilising by reso..
the -ve charge on carbon goes into the vacant d in Cl
Well I use inductive effect for quick consideration of acidity/basicity(if not an aryl or conjugated system). C-H bond is not considered that way as inductive effect is decided wrt H. We consider C-H electronegativity difference to be our reference electronegativity difference. A difference more than C-H bond means the other group has -I effect, and less than C-H bond means +I effect. It's that simple.
You can also see that after removal of proton the inductive effects of Cl stabilise the negative charge on C.
Inductive effect of halogens is far greater than their resonance effects...recall from ortho-para directing but deactivating character of halogens.