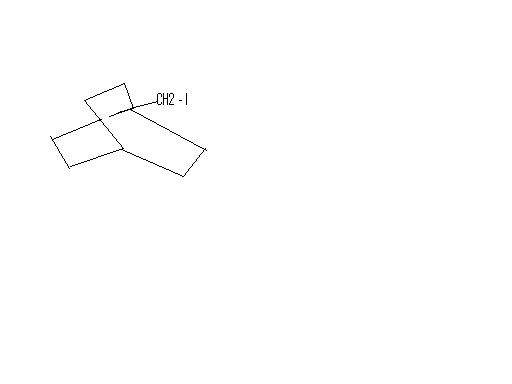
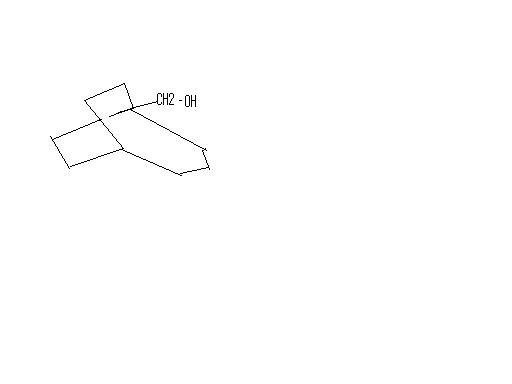
thats what even i got..but thats not the ans given .
9 Answers
Pritish Chakraborty
·2009-10-27 02:21:07
In place of Iodine you will get -OH group, and Ag will abstract the halogen atom and form a precipitate. AgI ppt is insoluble in ammonia solution of any concentration, that's how you test for it.
Thus you will get HNO3 and AgI as byproducts.
Pritish Chakraborty
·2009-10-27 02:34:56
Well the carbocation cannot rearrange to a secondary one as the bridge head carbon cannot be planar. But Ag will leave primary carbocations with no mercy, the mechanism will go forward. Can you post the ans?
Pritish Chakraborty
·2009-10-27 02:41:52
Maine toh yahi kaha tha?? iodine ke jagah -OH?
Ahh..wahan pe rearrangement kaise hua?