Answers are a , b and d.
Thanks all!! :P
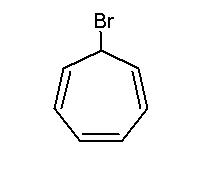
The above compound is treated with AgNO3 to form (X) . Which statement is true anout the product :
a) Product is aromatic.
b) Product has high dipole moment.
c) Product has less resonance energy.
d) Product is soluble in polar solvent.
Answer and also the complete Reaction mechanism please.
The answer is indeed (a). The intermediate obtained is the tropylium cation, a carbocation which is highly stable due to aromaticity.
Silver has a high affinity for halide ions. So it abstracts the bromide ion, irrespective of whether the resulting cation is stable or not (excluding the case of the benzene ring). A precipitate is formed (AgBr), which drives(forces) the reaction equilibrium forward to completion.
sorry pritish BUT your answer is wrong. the answer given is something else... answer later
well it may or may not occur to you . then answer given maybe WRONG ..
in fact pritish is very much correct . tropylium cation (aromatic) is formed