106
106NCERT and CAREY say that with nitration and sulphonation of aniline, meta and para products are obtained in an almost 50-50 ratio.
But as temperature is not applied to the second step, i think the product should be
2,4,6 -tribromo, 3-sulphonyl aniline (sorry for wrong nomenclature)
ABhi look at answr for first one #7
 1
1isnt fluorine most deactivating among halogens
 11
11Ans 6) I think that must be bcoz of the fact that in aromatic substitution S N reacn, the C- X bond is not cleaved at r.d.s. Hence it cannot affect the reactivity.
The carbon ion intermediate is stabilized by the -I effect of halogens and hence Cl is most reactive and I is least reactive.
 106
106yeah but y o>p
in electrophilic subs,
both o,p are more deactivating naa?
 11
11dunno if correct but acc to me :-
The groups which are deactivating in electrophilic substitution will be activating in nucleophilic substitution. E.W.G will be activating groups. The activating groups must be ortho and para w.r.t the leaving group.
ALso, the -M effect of NO2.
 106
106yeah but y??
I thot since there is max -I and -R for ortho, the carbanion TS is most stabilised.. then -para and finaly meta..
but y ortho>para ??
 11
11Ans 5) Not sure but ortho > para > meta ???
 106
106Q5, Q6 
replace the Q1 by Q5 and Q2 by Q6. pls
 1
14) Y should preferably be :
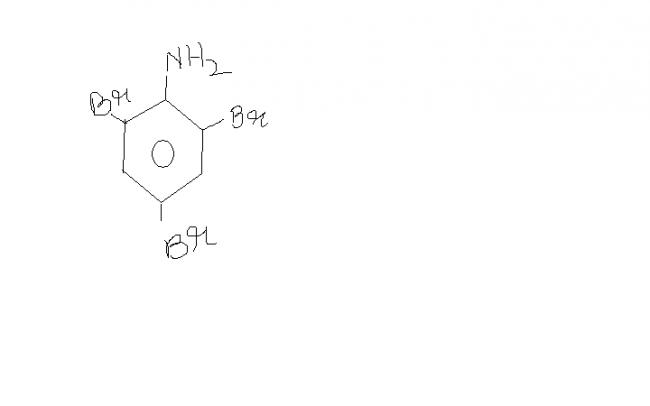
also , SO2 gas would be released
it would hv been better if heat would hv been mentioned.
 23
23bonne annee Q2 reason i hav given it earlier itself in #4
 106
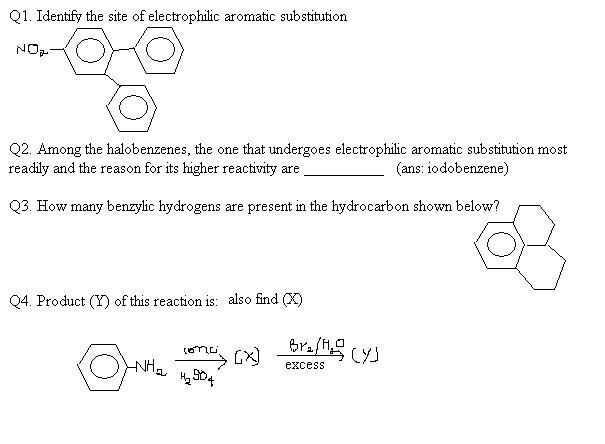
106strangely ans 1. was given to be on the 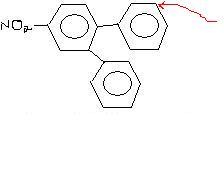
Q2. solomons mentions fluorobenzene
Q3. debo correct.. an explanation pls
Q4. What is (Y) ?
 19
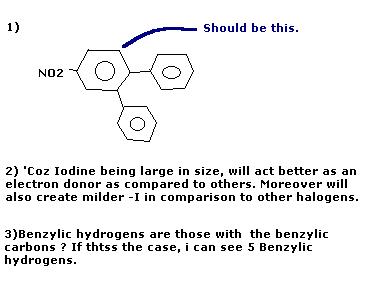
19ans 3 ) looks to be 5
ans 4) sulphanilic acid
ans1) correct answers on both occasions ! [1]
 11
11Ans 1) 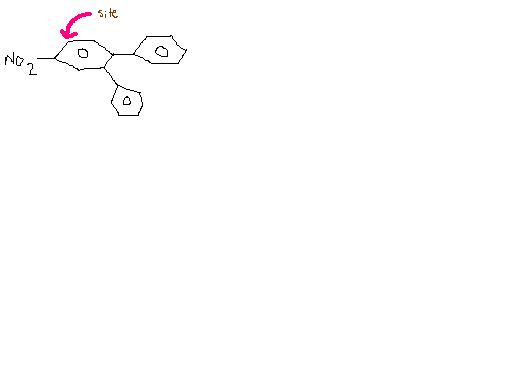
Ans 2) The electron withdrawing effect of the halogens dec considerably form F to I.
Ans 3) 4
Ans 4) X → Sulphanilic acid
 23
23avik u said that Iodine will be a good e- donor ....
but as compared to the other smaller halogens, Iodine is bigger in size, and it will be difficult for delocalisation to occur as the orbital overlap would be less effective than that with smaller halogens ......
and it is for the same reason that fluorine is the best o - p director among the halogens ...
 13
134) X will be tht Anilinium ion....n Br2/H2O will form HBr + HOBr... so...thinking over Y...