the alkene is symmetrical ? .. and its a cycloalkene ?
an alkyl halide of molecular formula C_5H_11Cl on refluxing with EtOH gives 2 cmpds: A & B.
A decolorises Br water. B does not . A on ozonolysis gives acetone & acetaldehyde. B on reaction with Na gives Hydrogen. It gives white ppt with with AgNO_3 soln. Elucidate the structure of the alkyl halide.
do u thnk this q is structured properly ? im getting A to be an alkene & B is an alcohol. what structure do i elucidate ? the only alkyl halide is C_5H_11Cl.
-
UP 0 DOWN 0 0 22

22 Answers
dont worry about room temperature or other things.. basically nothing is known about that...
lol.. gr8.. i hope u understood.. inspite of the most stupid chemistry that i have :)
btw on a cyclo molecule there would have been 2 oxygen linked carbon atoms.. in acetone there is only one!!
See bcos only one molecule is being formed u have started to hink that it could be a cycloalkene..
this is a very good guess..
but the product is acetone!!
so there could be one more solution.. when both the products formed are the same?!!
That is the case we are dealing here
that is what.. it has to be symmetric bcos there is only one product formed!
lol ! .. i didnt even read ur whole q properly ... :( .. i thght u just changed C_6 im doing it
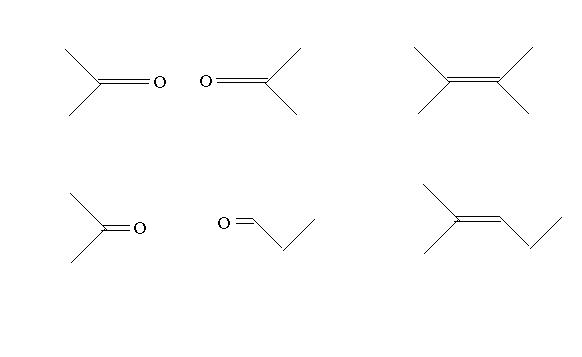
How will you decide which of these are the structures if u din find A?
A is 3-methyl 2-butene...
This part seems obvious to me...
now the alkyl halide will be easy to find using this alone?
u are finding them to get marks :D
basically ur point is exactly correct for the above problem..
but dont u think u will have to find A and B to get the answer to my problem?
so y are we finding A & B ? .. and wt do i elucidate ? .. no mechanism remains after i say that SN1 reaction mechanism ... so why shud i find A & B ? .. lol .. chem is the worst subject ive ever come across ! :(
btw
i will give u another question to explain ur problem exactly
an alkyl halide of molecular formula C_6H_13Cl on refluxing with EtOH gives 2 cmpds: A & B.
A decolorises Br water. B does not . A on ozonolysis gives acetone only. B on reaction with Na gives Hydrogen. It gives white ppt with with AgNO_3 soln. Elucidate the structure of the alkyl halide.
so here u will need to knwo A and B right?
If yes.. then u have clear idea of the question adn u dont need to let organic get into ur nerves :D
yes .. but i can find it out from the given info na ? the reaction proceeds via SN1 reaction. and its given that it gives ppt with AgNO_3 ... and the reaction takes place immediately .. so its a Tert halide ? wts the use of finding A & B ?
like it could be 1 chloro pentane
or 2 chloro pentane
or 2chloro 2methy butane
no no there are too many isomers.. right
so u need to find the exact isomer :)
but isnt C_5H_11Cl the alkyl halide ? wt other alkyl halide can u find ?
and then why do i find A & B if im not interested in their structures ?