dats a tedious n tym consuming job aragorn... less tym is required to search in d same thread, i think...!!!
I KEPt THE NAME OF THE QUESTION AS SUCH BECAUSE I WILL KEEP ON POSTING MY QUESTIONS IN THIS TAG ONLYYYYYYYY I WILL ADD NEW QUESTIONS DAILYYYYYYYYYYYY whats more than organic in chemistry.. so i will start with that
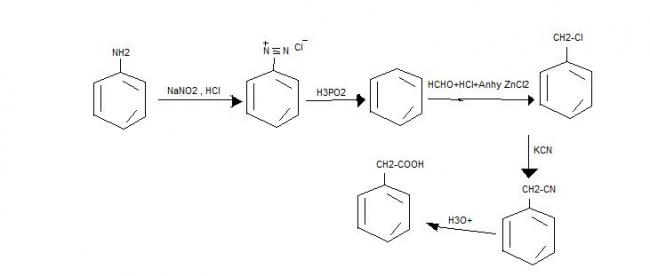
1]Convert aniline to phenyl acetic acid in NOT MORE THAN 5 steps....
2)Convert cyclopent2ene1one to cyclobutanol in NOT MORE THAN 5 steps....
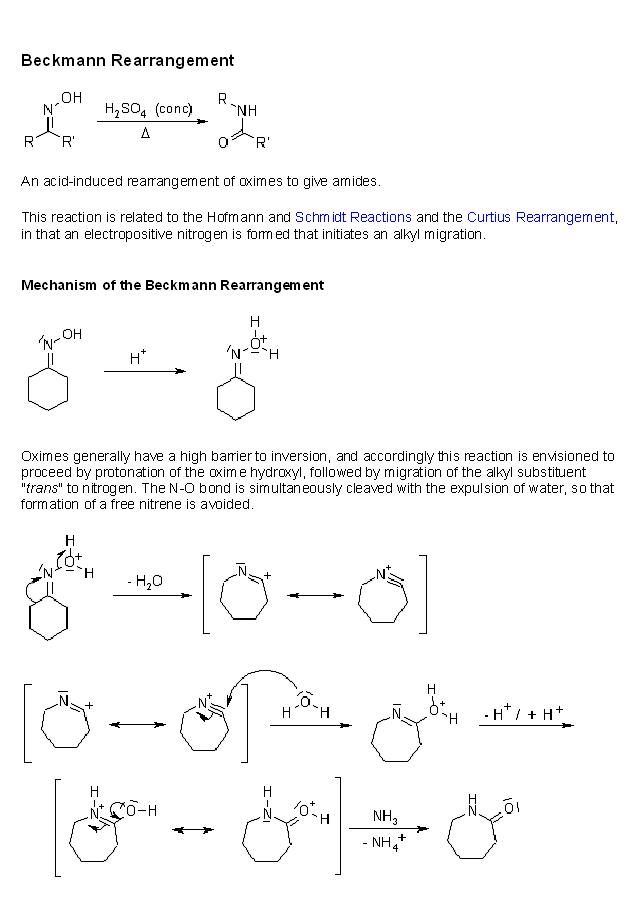
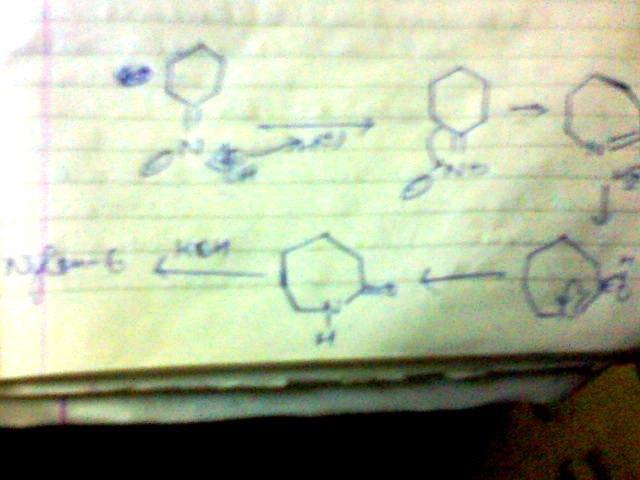
3]give the steps for the mechanism for the conversion of cyclohexanone oxime in the presence of H+, heat to caprolactam
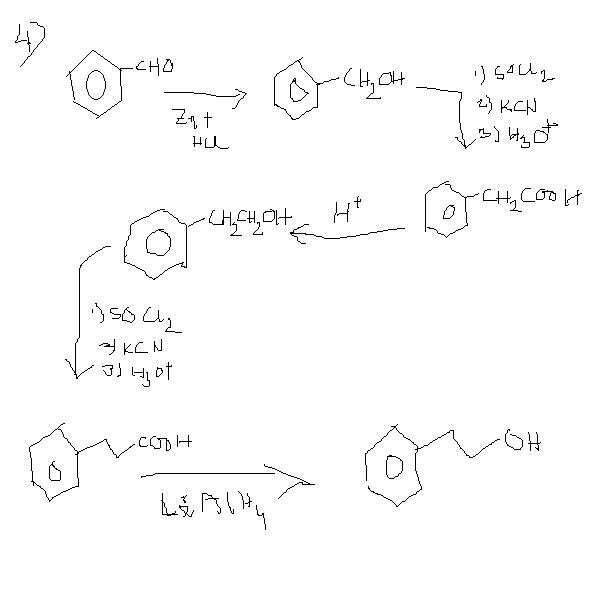
4]convert benzaldehyde to 3-phenylpropan-1-ol
5]what is fugisity
-
UP 0 DOWN 0 9 213

213 Answers
28 Two C4H6 isomers give the same C4H8O product from HgSO4 catalyzed hydration in aqueous acid.
However, these isomers give different C4H6Br4 products with excess bromine.
What are these isomeric hydrocarbons?
A) cyclobutene and methylenecyclopropane
B) 1,2-butadiene and 1,3-butadiene
C) 1-butyne and 2-butyne
D) 2-butyne and cyclobutene
30 Two C5H8 isomers undergo catalytic (Pt) hydrogenation to form the same C5H10 product. On ozonolysis followed by oxidative workup (H2O2), one isomer gave a C5H8O4 diacid, while the other isomer gave a C5H8O3 ketoacid.
Which of the following isomeric pairs correspond to this evidence?
A) cyclopentene and 1-pentyne
B) cyclopentene and 1-methylcyclobutene
C) 1-methylcyclobutene and 3-methylcyclobutene
D) cyclopentene and 3-methylcyclobutene
--------------------------------------------------------------------------------
31 Considering that the angles of a regular pentagon are 108º, why is cyclopentane not planar?
A) all the carbons are sp2 hybridized, so there is considerable angle strain.
B) The C-C bonds are formed by overlap of p-orbitals, so the 90º angle results in large angle strain.
C) The cyclic overlap of bonding orbitals results in anti-aromaticity destabilization.
D) The five C-C bonds have eclipsing strain.
--------------------------------------------------------------------------------
32 Which reaction conditions would best convert 3-hexyne to cis-3-hexene?
A) Pt catalyst and H2.
B) Lindlar's Pd catalyst and H2.
C) Na in liquid NH3.
D) NaNH2 in liquid NH3.
--------------------------------------------------------------------------------
33 Reaction of 1-hexene with NBS (N-bromosuccinimide) forms two isomeric bromohexenes, one of which is 3-bromo-1-hexene.
Which of the following is the other isomer?
A) 1-bromo-2-hexene.
B) 6-bromo-1-hexene.
C) 1-bromo-1-hexene.
D) 2-bromo-1-hexene.
--------------------------------------------------------------------------------
34 A C6H10 hydrocarbon forms an insoluble silver salt when treated with silver nitrate in ethanolic ammonia.
Acid catalyzed hydration with a HgSO4 catalyst generates a single C6H12O ketone, and pemanganate oxidation yields a C5H10O2 carboxylic acid
This compound is most likely which of the following?
A) cyclohexene.
B) methylenecyclopentane.
C) 1-hexyne.
D) 3-hexyne.
--------------------------------------------------------------------------------
35 Peroxide induced reaction of carbon tetrachloride with 1-butene produces which of the following?
A) 1,1,1,3-tetrachloropentane.
B) 1,1,1,2-tetrachloropentane.
C) 1-chloro-2-trichloromethylbutane.
D) 1,1-dichloro-2-ethylcyclopropane.
--------------------------------------------------------------------------------
36 Which of the following isomeric hexenes will have the smallest heat of hydrogenation?
A) 4-methyl-1-pentene.
B) (E)-4-methyl-2-pentene.
C) (Z)-4-methyl-2-pentene.
D) 2-methyl-2-pentene.
--------------------------------------------------------------------------------
37 A C8H14 hydrocarbon (X) is reduced by sodium in liquid ammonia to a single C8H16 product (Y).
Both of these compounds undergo hydrogenation (Pt catalyst) to give 2,5-dimethylhexane.
Ozonolysis of Y with an oxidative workup produces a single C4H8O2 carboxylic acid.
Reaction of Y with perbenzoic acid (C6H5CO3H) gives a chiral C8H14O product, but reaction with bromine gives an achiral C8H14Br2 product
What are X and Y?
A) X is 2,5-dimethyl-3-hexyne ; Y is cis-2,5-dimethyl-3-hexene.
B) X is 2,5-dimethyl-3-hexyne ; Y is trans-2,5-dimethyl-3-hexene.
C) X is 2.5-dimethyl-1,5-hexadiene ; Y is 2,5-dimethyl-3-hexyne.
D) X is 2,5-dimethyl-2,4-hexadiene ; Y is cis-2,5-dimethyl-3-hexene.
28 Which of the following procedures would be best for preparing isobutylisopropylamine, (CH3)2CHNHCH2CH(CH3)2?
A) (CH3)2CHBr + (CH3)2CHCH2NH2
B) (i) (CH3)2CHBr + (CH3)2CHCONHNa (ii) LiAlH4 in ether
C) (i) (CH3)2CHNH2 + (CH3)2CHCH=O (ii) H2 & Pt
D) (CH3)2CHCN + (CH3)2CHNH2
--------------------------------------------------------------------------------
29 What is the chief product from the Hofmann elimination of 4-methyl-2-aminopentane?
A) 4-methyl-1-pentene
B) 4-methyl-2-pentene
C) 2-methyl-1-pentene
D) 2-methyl-2-pentene
--------------------------------------------------------------------------------
30 Which of the following procedures would be best for preparing dimethylcyclohexylamine, C6H11N(CH3)2?
A) (i) dimethylamine + cyclohexanone (ii) NaBH3CN in methanol
B) dimethylamine + cyclohexylbromide in ether
C) cyclohexylamine + 2 CH3I in ether
D) (i) cyclohexylbromide + NaCN in methanol (ii) 2 CH3Li in THF
--------------------------------------------------------------------------------
31 What reagent would be best for converting the amide of (R)-2-phenylpropanoic acid, C6H5CH(CH3)CONH2, into (R)-1-amino-2-phenylpropane?
A) excess H2 & Pt.
B) NaOBr in aqueous base.
C) NaBlH4 in methanol
D) LiAlH4 in ether.
--------------------------------------------------------------------------------
32 What reagent would be best for converting the amide of (R)-2-phenylpropanoic acid, C6H5CH(CH3)CONH2, into (R)-1-phenylethylamine?
A) excess H2 & Pt.
B) NaOBr in aqueous base.
C) NaBlH4 in methanol
D) LiAlH4 in ether.
Both are easy. Similar processess for both:
1) take two beakers, one with the compound you have and the other with the compund u want
2) take a magician's hat and keep the beaker with the required product in it
3) assemble some friends to whom you want to show the conversions
4) widen your eyes and look up towards the sky and say "look! there's a white crow!"
5) In the fraction of the second that they look up, exchange the beakers
Your conversion is now complete in 5 steps.....
IMPORTANT NOTE:
Don't keep any harmful acids near you.......... cos your friends will take it and pour it on your face [3]
P.S:
This reply is not to be taken seriously. I know b555 won't... cos he thinks all of my replies are jokes [4]
it is just the project.... my friend does the same test to analyse the cations present in the coins... it is just to analyse or fiind the cations...
buddy c) is just the preparation of nylon-6......
this is beckmann rearrangement.....
yaar my answers should be pinked...
tell me if my answers r wrong b555
5)
Weaker the base better the leaving group, due to which RCOCl is more reactive than RCONH2 , this property of leaving group is known as (FUGISITY)
siddharth's is correct only...
am getting the same thing..
n its the shortest... i hope..
yes for beckmann,,,, only funda is ... the group trans to -OH will migrate...
hey i have given the answer of ur (5) part also just check it out...
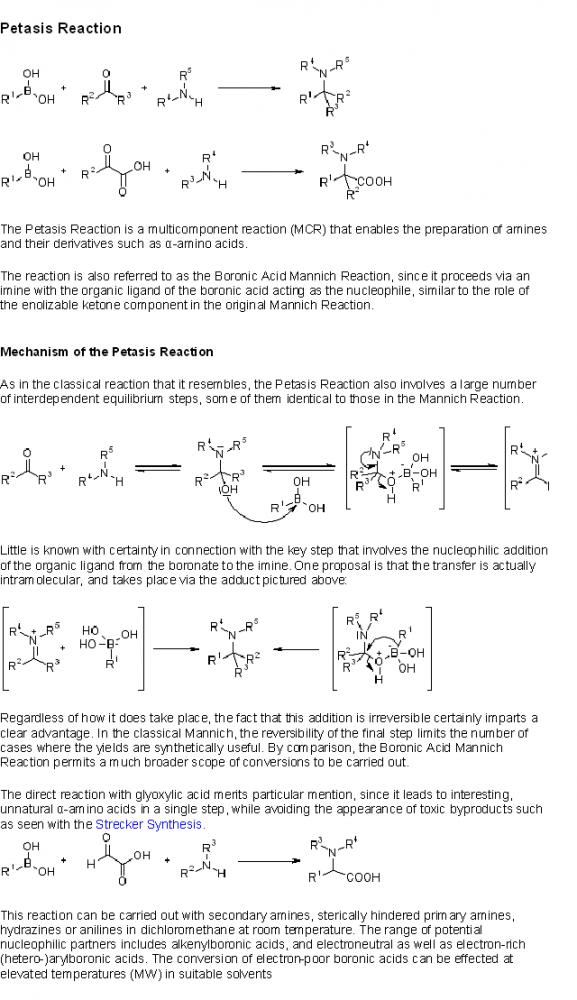
HEY GUYS PETASIS REACTION IS ALSO CALLED MANNICH REACTION??????????????