23]Why is monobromination of aromatic ring observed when the ring is treated with Br2 in presense of CS2?
I KEPt THE NAME OF THE QUESTION AS SUCH BECAUSE I WILL KEEP ON POSTING MY QUESTIONS IN THIS TAG ONLYYYYYYYY I WILL ADD NEW QUESTIONS DAILYYYYYYYYYYYY whats more than organic in chemistry.. so i will start with that
1]Convert aniline to phenyl acetic acid in NOT MORE THAN 5 steps....
2)Convert cyclopent2ene1one to cyclobutanol in NOT MORE THAN 5 steps....
3]give the steps for the mechanism for the conversion of cyclohexanone oxime in the presence of H+, heat to caprolactam
4]convert benzaldehyde to 3-phenylpropan-1-ol
5]what is fugisity
-
UP 0 DOWN 0 9 213

213 Answers
22]. What do you mean by 'Complete Delocalisation of Electrons', a neccesity for aromaticity by Huckel's rule? Is it possible to judge from the structure of a compound whether complete delocalisation of electrons is available or not?
General Selection Problem
26. Which of the following is true for 3-methylbutanal?
a) This compound may be classified as an aldehyde.
b) This compound may be classified as a ketone.
c) An aldol reaction takes place on treatment with NaOH solution.
d) There is no reaction with LiAlH4 in ether solution.
e) An excess of CH3MgBr in ether reacts to give 4-methyl-2-pentanol.
f) Wolff-Kishner reduction gives butane.
g) This compound is an isomer of 3-pentanone.
--------------------------------------------------------------------------------
27. Which of the following is true for 3-methyl-2-butanone?
a) It may be prepared by CrO3 oxidation of 2-methyl-2-butanol.
b) Reaction with NaBH4 gives a secondary alcohol.
c) It may be prepared by acidic Hg2+ catalyzed hydration of 3-methyl-1-butyne.
d) It forms a silver mirror on treatment with Ag(NH3)2.
e) This compound is an isomer of 4-penten-1-ol.
33 Which of the following statements is true for a pair of diastereomers?
A) they will have identical physiological properties.
B) they will have specific rotations of opposite sign.
C) they will have identical chemical properties (e.g. reactivity)
D) they will have different physical properties.
--------------------------------------------------------------------------------
34 Which of the following descriptive terms would never be applied to a pair of stereoisomers ?
A) enantiomers
B) tautomers
C) diastereomers
D) epimers
--------------------------------------------------------------------------------
35 What kind of reagent would be needed to resolve a racemic amine, such as 2-aminobutane ?
A) the pure optically active amine to serve as a template for crystallization.
B) an achiral carboxylic acid to give a racemic mixture of amine salts.
C) an enantiomerically pure chiral carboxylic acid to give a diastereomeric mixture of amine salts.
D) a racemic chiral carboxylic acid to give a complete mixture of isomeric amine salts.
--------------------------------------------------------------------------------
36 What common symmetry elements if any are found in the stable chair conformer of trans-1,4-dichlorocyclohexane?
A) a single mirror plane and a C2 rotational axis.
B) a C3 rotational axis but no mirror plane.
C) two orthogonal mirror planes and a C2 rotational axis.
D) a single C2 rotational axis but no mirror plane.
--------------------------------------------------------------------------------
37 What common symmetry elements if any are found in the stable chair conformer of trans-1,2-dichlorocyclohexane?
A) a single mirror plane and a C2 rotational axis.
B) a single mirror plane and a C3 rotational axis.
C) two orthogonal mirror planes and a C2 rotational axis.
D) a single C2 rotational axis but no mirror plane.
--------------------------------------------------------------------------------
38 Reaction of 3-methyl-1,4-cycloheptadiene with excess perbenzoic acid (C6H5CO3H) forms a diepoxide product.
How many stereoisomers, counting enantiomers, are expected from this reaction ?
A) 4 (two pairs of enantiomers)
B) 4 (two meso compounds and a pair of enantiomers)
C) 5 (two pairs of enantiomers and a meso compound)
D) 6 (two pairs of enantiomers and two meso compounds)
--------------------------------------------------------------------------------
39 The antimalarial alkaloid quinine, C20H24N2O2, is optically active.
An ethanol solution of 8g quinine in 100mL displays a rotation of -13.6º in a 1dm polarimeter tube.
What is the specific rotation of quinine?
A) -85º
B) -170º
C) -43º
D) -26º
--------------------------------------------------------------------------------
--------------------------------------------------------------------------------
41 Which of the following compounds has a prochiral methylene group (i.e. the hydrogen atoms are diastereotopic)?
A) propane , CH3CH2CH3
B) cyclopropane , (CH2)3
C) 2-methylpropene , CH2=C(CH3)2
D) ethanol , CH3CH2OH
--------------------------------------------------------------------------------
42 The thermal electrocyclic closure of (2E,4Z,6E)-2,4,6-octatriene gives which of the following?
A) cis-5,6-dimethyl-1,3-cyclohexadiene
B) trans-5,6-dimethyl-1,3-cyclohexadiene
C) cis-3,6-dimethyl-1,4-cyclohexadiene
D) cis-3,6-dimethyl-1,4-cyclohexadiene
--------------------------------------------------------------------------------
43 The Cope rearrangement, [3,3]-sigmatropic shift, of meso-3,4-dimethyl-1,5-hexadiene gives which of the following?
A) 1,7-octadiene
B) (2Z,6Z)-2,6-octadiene
C) (2E,6Z)-2,6-octadiene
D) (2E,6E)-2,6-octadiene
49 Which of the following statements is not an essential feature of an optically active compound?
A) the molecules of an optically active compound will be dissymetric or asymmetric.
B) the molecules of an optically active molecule must have at least one stereogenic site.
C) an optically active compound's molecular configuration will not be identical with its mirror image.
D) an optically active compound will have at least one stereoisomer.
--------------------------------------------------------------------------------
50 Which of the following statements is not correct?
A) a pair of enantiomeric compounds will have the same melting point.
B) a pair of enantiomeric compounds will have the same solubility in ethanol.
C) a pair of enantiomeric compounds will have exactly the same functional groups.
D) a pair of enantiomeric compounds will have identical optical rotations.
General and Miscelleneous
1. How many electrons occupy the bonding molecular orbitals of a CN triple bond?
A) 2 B) 4 C) 6 D) 8
--------------------------------------------------------------------------------
2. Which of the following species is not amphoteric ?
A) NH3 B) HF C) NH4(+) D) HCO3(-)
--------------------------------------------------------------------------------
3 What is the relationship of the two structures shown below?
A) they are identical.
B) they are conformations of the same structure
C) they are constitutional isomers.
D) they are different compounds with different compositions
--------------------------------------------------------------------------------
4 Which of the following molecules will not have a dipole moment?
A) CH3Cl
B) CH3OCH3
C) CH2Cl2
D) CCl4
--------------------------------------------------------------------------------
5 Which of the following molecules has a dipole moment?
A) CBr4
B) CH2=CH2
C) BF3
D) SO2
--------------------------------------------------------------------------------
6 Which of the following molecules has a linear shape?
A) NH3
B) H2S
C) CO2
D) H2CO
--------------------------------------------------------------------------------
7 Which of the following intermediates is pyramidal in shape?
A) H3C(+)
B) H2C:
C) H3C:(-)
D) HC≡C:(-)
--------------------------------------------------------------------------------
8 In which compound does carbon have the highest oxidation state?
A) CH4
B) HCN
C) H2CO
D) CH2Cl2
--------------------------------------------------------------------------------
9 Which of the following statements applies to a C10H14O2 compound?
A) it may have 2 double bonds and 2 rings
B) it may have 3 double bonds and 0 rings
C) it may have 1 triple bond and 3 rings
D) it may have 0 double bonds and 3 rings
12 Which of the following compounds would be considered an electrophilic reagent?
A) NH3
B) Br2
C) CH3OH
D) NH2OH
--------------------------------------------------------------------------------
13 Which of the following compounds would you expect to be most soluble in water?
A) CH2Cl2
B) C6H12 (cyclohexane)
C) CH3CH2OH
D) C2H5OC2H5
--------------------------------------------------------------------------------
14 The following compounds have similar molecular weights. Which has the highest boiling point?
A) CH3CH=O
B) C2H5OH
C) CH3OCH3
D) CH3CH2CH3
--------------------------------------------------------------------------------
15 Which of the following compound pairs are constitutional isomers?
A) CH3CH2OCH3 and CH3CH2CHO
B) CH3CH2CHO and CH3CH2CH2OH
C) CH3COCH2CH3 and CH3CH2COCH3
D) CH3CH2CH2CHO and CH3COCH2CH3
--------------------------------------------------------------------------------
16 Which of the following compounds may be classed as a protic solvent?
A) tert-butanol
B) diethyl ether
C) n-hexane
D) acetone
--------------------------------------------------------------------------------
17 Classify the following reaction.
A) substitution
B) addition
C) elimination
D) rearrangement
--------------------------------------------------------------------------------
18 Which of the following is not a nucleophile?
A) H2O
B) CH3NH2
C) C2H5SH
D) C6H12 (cyclohexane)
--------------------------------------------------------------------------------
19 Which of the following is not an electrophile?
A) C2H5OC2H5
B) BF3
C) [CH3]3C(+)
D) HOCl
--------------------------------------------------------------------------------
21 Which of the following compounds has a C-H bond with the lowest bond dissociation energy?
A) C2H6
B) C6H6
C) C2H2
D) CH3CH=CH2
--------------------------------------------------------------------------------
22 In order for a reagent to behave as a nucleophile it must have ...
A) an overall positive charge.
B) an overall negative charge.
C) a non-bonding electron pair.
D) a nitrogen or sulfur atom.
--------------------------------------------------------------------------------
23 The H–C–O bond angle in H2C=O (formaldehyde) is approximately .....
A) 90º
B) 109º.
C) 120º.
D) 180º.
42 How would the bond strength of the C:C double bond in an alkene compare to that of a C:C single bond in the corresponding alkane?
A) The double bond would have the same strength as the single bond.
B) The double bond would be stronger than, but less than twice as strong as the single bond.
C) The double bond would have exactly twice the strength of the single bond.
D) The double bond would have more than twice the strength of the single bond.
--------------------------------------------------------------------------------
43 Which of the following cations is most stable?
A) FH2+
B) OH3+
C) NH4+
D) CH5+
--------------------------------------------------------------------------------
44 The toxic environmental pollutant dioxin is 44.8% carbon, 1.25% hydrogen and 44.0% chlorine. Its molecular weight is 320±10
What is the molecular formula of dioxin?
A) C10H8O4Cl4
B) C12H6O2Cl4
C) C6H2OCl2
D) C12H4O2Cl4
--------------------------------------------------------------------------------
45 Which of the following covalent compounds does not have any formally charged atoms?
A) (CH3)3NO
B) CH2=N=N
C) CH3-O-N=O
D) CH3C≡NO
--------------------------------------------------------------------------------
46 Which of the following statements is not generally correct?
A) endothermic reactions have larger energies of activation than exothermic reactions
B) the rate of a reaction is proportional to its activation energy
C) the rate of a reaction generally increases as the temperature is raised
D) all reactions in which bonds are broken and formed have a significant activation energy
--------------------------------------------------------------------------------
47 Which of the following compounds has no isomer?
A) CH3CH2CH2Cl
B) CH3CHO
C) CH2=CHCl
D) ClCH2CH2Cl
--------------------------------------------------------------------------------
48 Which of the following statements about carbon is not correct?
A) carbon forms strong covalent bonds to itself, allowing chains and rings to be made.
B) carbon expands its valence shell to accomodate more than eight electrons and thus forms double and triple bonds.
C) carbon forms strong covalent bonds to elements like nitrogen and oxygen because it does not have lone pairs of valence electrons to destabilize the bonds.
D) carbon and hydrogen have similar electronegativity and form strong bonds to each other, thus avoiding the high reactivity shown by metal hydrides.
16 Which of the following C6H12 isomers has the largest heat of combustion?
A) cyclohexane
B) methylcyclopentane
C) ethylcyclobutane
D) propylcyclopropane
--------------------------------------------------------------------------------
17 The radical halogenation of 2-methylpropane gives two products: (CH3)2CHCH2X (minor) and (CH3)3CX (major)
Chlorination gives a larger amount of the minor product than does bromination, Why?
A) Bromine is more reactive than chlorine and is able to attack the less reactive 3º C-H.
B) Bromine atoms are less reactive (more selective) than chlorine, and preferentially attack the weaker 3º C-H bond.
C) The methyl groups are more hindered to attack by the larger bromine atom.
D) Bromination is reversible and the more stable 3º-alkyl bromide is formed exclusively.
--------------------------------------------------------------------------------
18 A tertiary carbocation (carbonium ion) is more stable than either a secondary or primary carbocation because ...
A) it carries three positive charges
B) it has a pyramidal configuration
C) it has a trigonal planar configuration
D) it possesses three electron-donating substituent groups
--------------------------------------------------------------------------------
19 Which of the following is not an electrophile?
A) C2H5OC2H5
B) BF3
C) [CH3]3C(+)
D) HOCl
--------------------------------------------------------------------------------
20 Which of the following olefins would you expect to react most rapidly with concentrated sulphuric acid ?
A) H2C=CH2
B) (CH3)2C=CH2
C) Cl2C=CCl2
D) CF3CH=CH2
--------------------------------------------------------------------------------
21 Which compound is a likely product from addition of Cl2 to 1-butene?
A) CH3CH2CH2CHCl2
B) CH3CH2CHClCH2Cl
C) ClCH2CH2CH2CH2Cl
D) CH3CH2CCl2CH3
--------------------------------------------------------------------------------
22 The product from OsO4 hydroxylation of trans-2-butene will be ...
A) achiral
B) optically active
C) racemic
D) a meso compound
--------------------------------------------------------------------------------
23 The product from bromine addition to trans-2-butene will be ...
A) optically active
B) racemic
C) a meso compound
D) chiral
--------------------------------------------------------------------------------
24 Addition of 1 equivalent of bromine to 2,4-hexadiene at 0º C gives 4,5-dibromo-2-hexene plus an isomer.
Which of the following is that isomer?
A) 5,5-dibromo-2-hexene
B) 2,5-dibromo-3-hexene
C) 2,2-dibromo-3-hexene
D) 2,3-dibromo-4-hexene
--------------------------------------------------------------------------------
25 How many sp hybridized carbon atoms are present in a molecule of 3-methyl-4-vinyl-1,2-heptadien-5-yne?
A) 2
B) 3
C) 4
D) 5
--------------------------------------------------------------------------------
26 Treatment of 1-methylcyclohexene with an ether solution of diborane ( B2H6 ), followed by reaction with alkaline H2O2 produces what product?
A) 1-methylcyclohexanol
B) cis-1-methylcyclohexane-1,2-diol
C) cis-2-methylcyclohexanol
D) trans-2-methylcyclohexanol
43 Reaction of 1,1-dibromopentane with three equivalents of NaNH2 in ether is followed by treatment with 0.1M HCl at 0º C.
What is the product?
A) cyclopentene.
B) 1,2-pentadiene.
C) 2-pentyne.
D) 1-pentyne.
--------------------------------------------------------------------------------
44 Which of the following reagents and conditions would best serve to convert 1-butyne to 1,1-dibromobutane?
A) 2 equivalents of HBr, no peroxides.
B) 2 equivalents of HBr, with peroxides.
C) 1 equivalent of Br2.
D) 2 equivalents of Br2 followed by i equivalent of KOH.
--------------------------------------------------------------------------------
45 What is the relative rate of addition of HBr to I: 1,3-pentadiene; II: 1,4-pentadiene; and III: 1-pentyne?
A) I > II > III.
B) III > II > I.
C) II > I > III.
D) III > I > II.
--------------------------------------------------------------------------------
46 A chiral C6H12 hydrocarbon undergoes catalytic hydrogenation to yield an achiral C6H14 product. What is the starting compound?
A) cis-2-hexene
B) 3-methyl-2-pentene
C) 4-methyl-2-pentene
D) 3-methyl-1-pentene
--------------------------------------------------------------------------------
47 Reaction of 3,3,6,6-tetramethyl-1,4-cyclohexadiene, first with excess aqueous mercuric acetate, then followed by sodium borohydride reduction, produces a mixture of isomeric C10H20O2 alcohols.
Excluding enantiomers, how many isomeric products may be formed in this reaction?
A) 2
B) 4
C) 6
D) 8
53 Which one of the following compounds contains the greatest number of sp2 hybridized carbon atoms?
A) 1,4-Cycloheptadiene
B) 2,5-dimethyl-2,3,4-hexatriene
C) Phenylacetylene
D) 1,1-diallyl-3,3-divinylcyclobutane
--------------------------------------------------------------------------------
54 Which one of the following compounds contains the greatest number of sp hybridized carbon atoms?
A) 1,4-Cyclooctadiene
B) 2,5-dimethyl-2,3,4-hexatriene
C) Phenylacetylene
D) 1,1-diallyl-3,3-divinylcyclobutane
--------------------------------------------------------------------------------
55 Which one of the following compounds contains the greatest number of sp3 hybridized carbon atoms?
A) 1,4-Cyclooctadiene
B) 2,5-dimethyl-2,3,4-hexatriene
C) Phenylacetylene
D) 1,1-diallyl-3,3-divinylcyclobutane
--------------------------------------------------------------------------------
56 Which of the following isomers has the lowest heat of combustion?
A) cis-1,2-dimethylcyclohexane
B) trans-1,2-dimethylcyclohexane
C) cis-1,3-dimethylcyclohexane
D) trans-1,3-dimethylcyclohexane
--------------------------------------------------------------------------------
57 Compound Z, C8H14, reacts with excess hydrogen and a Pt catalyst to give 2,5-dimethylhexane as the only product.
Z diplays three 13C nmr signals, all at higher field than 100 ppm, and does not absorb in the UV at wavelengths greater than 200 nm.
Oxidation of Z by either ozone or potassium permanganate produces a single C4H8O2 carboxylic acid. Deduce the structure of Z.
A) 2,5-dimethyl-3-hexyne
B) trans-2,5-dimethyl-3-hexene
C) cis-2,5-dimethyl-3-hexene
D) 2,5-dimethyl-2,4-hexadiene
8 Which of the following common names does not represent a dicarboxylic acid?
A) lactic acid
B) succinic acid
C) phthalic acid
D) glutaric acid
--------------------------------------------------------------------------------
9 Which of the following statements is not generally true?
A) the boiling point of a carboxylic acid is higher than that of its methyl ester.
B) methyl esters are more reactive acylating agents than their amide counterparts.
C) amide hydrolysis may be carried out with either strong acid or base catalysis.
D) Fischer esterification of acids with alcohols requires a strong base catalyst.
--------------------------------------------------------------------------------
10 What is the order of increasing acidity for the following compounds? (weaker < stronger) I 4-methylpentanoic acid II 3-chloropentanoic acid III 2-bromopentanoic acid IV 2,2-dichloropentanoic acid
A) I < II < III < IV
B) IV < III < II < I
C) I < III < II < IV
D) II < III < I < IV
--------------------------------------------------------------------------------
11 When comparing the acidity of propanoic acid and pyruvic acid, CH3COCO2H, which of the following statements is correct.
A) propanoic acid has a lower pKa and a smaller Ka than pyruvic acid
B) propanoic acid has a lower pKa and a larger Ka than pyruvic acid
C) propanoic acid has a higher pKa and a larger Ka than pyruvic acid
D) propanoic acid has a higher pKa and a smaller Ka than pyruvic acid
--------------------------------------------------------------------------------
12 Consider the following dicarboxylic acids? I adipic acid
HO2C(CH2)4CO2H II malonic acid
CH2(CO2H)2 III oxalic acid
(CO2H)2 IV succinic acid
HO2C(CH2)2CO2H
What is the order of increasing acid strength? (weaker < stronger)
A) I < II < III < IV
B) IV < III < II < I
C) I < IV < II < III
D) II < I < IV < III
--------------------------------------------------------------------------------
13 Dicarboxylic acids have two pKa's.
For maleic acid (cis-2-butenedioic acid) these are pKa1 = 2.0, and pKa2 = 6.3
For fumaric acid (trans-2-butenedioic acid) these are pKa1 = 3.0, and pKa2 = 4.5
Which factor best explains why the cis-isomer has a smaller pKa1 and a larger pKa2 than the trans-isomer?
A) intramolecular steric hindrance
B) intramolecular dipole repulsion
C) intramolecular hydrogen bonding
D) selective solvation in water
--------------------------------------------------------------------------------
14 An equimolar mixture of benzoic acid and benzyl alcohol is dissolved in equal volumes of ether and 5% aqueous NaOH.
The resulting mixture separates into two immiscible liquid layers. Which of the following is approximately correct?
A) both organic solutes are largely in the ether layer
B) the benzyl alcohol is in the ether layer and the benzoic acid is in the water layer
C) both organic solutes are largely in the water layer
D) the benzyl alcohol is in the water layer and the benzoic acid is in the ether layer
--------------------------------------------------------------------------------
15 Fischer esterification of phenylacetic acid with 1-propanol gave a mixture of 93% of the ester, propyl phenylacetate, contaminated with 7% unreacted acid.
Which of the following treatments would be best used to purify the ester?
A) reduce the unwanted acid with LiAlH4 in ether.
B) wash an ether solution of the crude product with concentrated brine (aq. NaCl).
C) wash an ether solution of the crude product with 5% aqueous sulfuric acid.
D) wash an ether solution of the crude product with 5% aqueous sodium carbonate.
--------------------------------------------------------------------------------
16 Which of the following would not be a useful reaction for preparing isobutyric acid, (CH3)2CHCO2H?
A) 2-methyl-1-propanol + Jones' reagent
B) 2-bromopropane + CO2; followed by hydrolysis
C) cis-2,5-dimethyl-2-hexene + O3; followed by H2O2
D) 2-bromopropane + NaCN; followed by acid-catalyzed hydrolysis
--------------------------------------------------------------------------------
17 Which of the following would not be a useful method for converting a carboxylic acid into an ester derivative?
A) RCO2H + CH2N2 in ether
B) RCO2H + (CH3)2C=CH2 & acid catalyst
C) RCO2H + C2H5OH & acid catalyst + heat (-H2O)
D) RCO2(-) Na(+) + (CH3)3CBr
--------------------------------------------------------------------------------
18 Which of the following is an intermediate in the Fischer esterification of propanoic acid with ethanol?
A) 1-propoxy-1,1-dihydroxyethane
B) 2-propoxy-1,1-dihydroxyethane
C) 1-ethoxy-1,1-dihydroxypropane
D) 2-ethoxy-1,1-dihydroxypropane
--------------------------------------------------------------------------------
19 Which of the following reagents does not react with benzoic acid, converting it into a different compound?
A) NaI in acetone
B) SOCl2
C) LiAlH4 in ether
D) excess CH3Li in pentane
--------------------------------------------------------------------------------
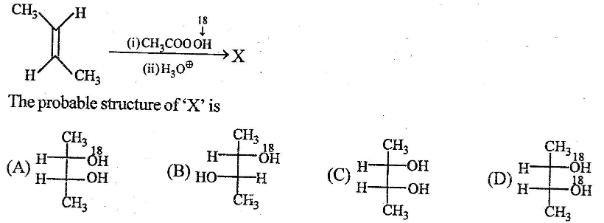
20 Which statement regarding isotope exchange of 18OH2 with the oxygen of a carboxyl group is correct?
no exchange occurs under any circumstances
base-catalyzed exchange is more effective than acid-catalyzed exchange
acid-catalyzed exchange is more effective than base-catalyzed exchange
acids and bases are equally effective in catalyzing an exchange
--------------------------------------------------------------------------------
21 Which of the following compounds could not be converted into pivalic acid ( 2,2-dimethylethanoic acid ) in three or fewer steps?
A) 2,2-dimethyl-1-butene
B) 2,3-dimethyl-2-butene
C) 2,2-dimethyl-1-propanol
D) 2-bromo-2-methylpropane
--------------------------------------------------------------------------------
22 Treatment of benzoic acid with excess 18OH2 and a strong acid catalyst results in what change?
A) both oxygens of the carboxyl group exchange with the 18O of the water
B) only the carbonyl oxygen exchanges with the 18O of the water
C) only the OH oxygen exchanges with the 18O of the water
D) no change takes place
--------------------------------------------------------------------------------
23 Two C8H9Br isomers form Grignard reagents which on carboxylation give isomeric carboxylic acids.
Oxidation of each isomeric acid with hot KMnO4 produces the same C9H6O6 tricarboxylic acid.
Which of the following compounds are the original isomeric bromides?
A) I & II
B) III & IV
C) I & IV
D) II & III
--------------------------------------------------------------------------------
24 Which of the following reagents will reduce a carboxylic acid to a 1 º-alcohol under mild conditions? I BH3 in ether II NaBH4 in ethanol III H2 & Pt catalyst IV LiAlH4 in ether
A) I & IV
B) II & III
C) only III
D) all of them
--------------------------------------------------------------------------------
25 What is the order of increasing base strength for the following salts? (weaker < stronger) I sodium ethoxide II potassium formate III sodium benzoate IV sodium dichloroacetate
A) I < II < III < IV
B) IV < II < III < I
C) II < III < IV < I
D) IV < I < II < III
27 Which compound does not react with ammonia to form propanamide under mild conditions?
A) propanoic acid
B) propanoyl chloride
C) ethyl propanoate
D) propanoic anhydride
--------------------------------------------------------------------------------
28 Which sequence of reactions would be best for the conversion of toluene into para-bromophenylacetic acid.? ( NBS = N-bromosuccinimide )
A) i) Br2 & FeBr3; ii) KMnO4 & heat; iii) NaCN in ethanol; iv) H3O(+) & heat
B) i) Br2 & FeBr3; ii) NBS in CCl4; iii) Mg in ether followed by CO2; iv) H3O(+)
C) i) Br2 & FeBr3; ii) NBS in CCl4; iii) NaCN in ethanol; iv) H3O(+) & heat
D) i) Br2 & FeBr3; ii) KMnO4 & heat; iii) Mg in ether followed by CO2; iv) H3O(+)
--------------------------------------------------------------------------------
29 On heating in the presence of bromine, 2,2-dimethyl-3-oxobutyric acid produces 3-bromo-3-methyl-2-butanone.
What unstable intermediate is involved in this reaction?
A) 1,2,2-trimethylcyclopropanol
B) 2,2-dimethyl β butyrolactone
C) 2-hydroxy-3-methylbutene
D) dimethylketene
--------------------------------------------------------------------------------
30 Fischer esterification of mesitoic acid ( 2,4,6-trimethylbenzoic acid ) is extremely slow compared with benzoic acid itself.
What is the major factor accounting for this difference in reactivity?
A) steric hindrance by the ortho methyl groups.
B) the electron donating inductive effect of the methyl substituents.
C) acid-catalyzed rearrangement of the methyl substituents.
D) rapid acid-catalyzed decarboxylation of mesitoic acid.
--------------------------------------------------------------------------------
31 Which of the following reagents would be best for reducing an ester to an aldehyde?
A) LiAlH4 in ether
B) B2H6 in ether
C) NaBH4 in aqueous ethanol
D) diisobutylaluminum hydride (DIBAH) in toluene at -78 ºC
--------------------------------------------------------------------------------
32 Which of the following reagents are suitable for reducing an amide to an aldehyde? I LiAlH4 in ether II diisobutylaluminum hydride (DIBAH) in toluene at -78 ºC
III LiAlH(t-C4H9O)3 in ether at -78 ºC IV NaBH4 in aqueous ethanol
A) I & IV
B) II & III
C) only I
D) all but III are suitable
--------------------------------------------------------------------------------
33 Which of the following reagents are suitable for reducing an acyl chloride to an aldehyde? I LiAlH4 in ether II NaBH4 in tert-butanol
III LiAlH(t-C4H9O)3 in ether at -78 ºC IV H2 and Pd/BaSO4 catalyst
A) III & IV
B) I & IV
C) only I
D) all are suitable
--------------------------------------------------------------------------------
34 Which of the following methods would not be useful for preparing ketones? I Friedel-Crafts reaction of an acyl chloride with benzene (AlCl3 catalysis)
II reaction of R2CuLi with an acyl chloride in ether at low temperature.
III reaction of Grignard reagents with nitriles, followed by hydrolysis
IV reaction of methyllithium with the lithium salt of a carboxylic acid, followed by hydrolysis
A) II & IV
B) II & III
C) only III
D) none (all are useful)
36 Which of the following reactions is most likely to produce ethyl propanoate?
A) sodium ethoxide + propanoic acid
B) propanol + acetyl chloride
C) sodium propanoate + acetic anhydride
D) potassium propanoate + ethyl iodide
--------------------------------------------------------------------------------
37 If diethyl amine is treated separately with the following derivatives of isobutyric acid, what order of reactivity is expected?
greater reactivity > lesser reactivity I isobutyronitrile
( 2-methylpropanenitrile ) II isobutyryl chloride
( 2-methylpropanoly chloride ) III ethyl isobutyrate IV isobutyric anhydride
( 2-methylpropanoic anhydride )
A) I > II > III > IV
B) II > IV > III > I
C) IV > II > III > I.
D) IV > III > II > I
--------------------------------------------------------------------------------
38 Methyl esters of carboxylic acids, RCO2CH3, have somewhat higher molecular masses than 1 º-amide, RCONH2, derivatives of the same acid.
However, the amides have much higher boiling points. What is responsible for this difference?
A) hydrogen bonding
B) resonance conjugation of N with C=O
C) the lower electronegativity of N versus O
D) rapid pyramidal inversion of the nitrogen
51 2,2-Dimethyl-1,3-cyclohexanedione is refluxed several hours in an aqueous dioxane solution of sodium hydroxide (10%).
The resulting solution is adjusted to pH=5 by addition of dilute HCl and then extracted with ether.
What compound has been prepared by this procedure?
A) 2-cyclohexenone
B) 5-oxohexanoic acid
C) 6-methylheptanoic acid
D) 5-oxo-6-methylheptanoic acid
--------------------------------------------------------------------------------
52 The malonic ester synthesis is useful for preparing substituted acetic acids.
Which of the following would not be easily prepared by this method? I phenylacetic acid II 3-phenylpropanoic acid III 2,2-dimethylpropanoic acid IV 4-pentenoic acid
A) IV
B) II & IV
C) I & III
D) all but I
--------------------------------------------------------------------------------
53 Which set of reaction conditions is best suited for the preparation of 2,2-dimethylpropanoic acid from 2-bromo-2-methylpropane?
A) 1. NaCN in ethanol; 2. H3O(+) & heat
B) 1. NaC≡CH in ether; 2. aqueous KMnO4 & heat
C) 1. Mg in ether; 2. CO2, then H3O(+)
D) 1. Mg in ether; 2. CH3CHO; 3. Jones' reagent
--------------------------------------------------------------------------------
54 Which set of reaction conditions is best suited for the preparation of 5-oxo-hexanoic acid from 5-bromo-2-pentanone?
A) 1. NaCN in ethanol; 2. H3O(+) & heat
B) 1. NaC≡CH in ether; 2. aqueous KMnO4 & heat
C) 1. Mg in ether; 2. CO2, then H3O(+)
D) 1. NaOH in ethanol; 2. Jones' reagent
--------------------------------------------------------------------------------
55 Which of the following is most readily decarboxylated on heating?
A) sodium 4-oxopentanoate
B) succinic acid
C) phthalic acid
D) 4-methyl-3-pentenoic acid
--------------------------------------------------------------------------------
56 Which one of the following compounds would react with C2H5MgBr to make 3-pentanol ?
A) ethanal
B) ethyl formate
C) acetic acid
D) acetone
--------------------------------------------------------------------------------
57 The acetoacetic ester synthesis is useful for preparing methyl ketones.
Which of the following would not be easily prepared by this method? I acetylcyclopentane II acetophenone III 3-ethyl-2-pentanone IV 3-methyl-4-phenyl-2-butanone
A) I & II
B) III & IV
C) only I
D) only II
--------------------------------------------------------------------------------
58 Devise a series of reactions to convert ethyl 3-oxobutyrate to ethyl 4-oxopentanoate.
Select reagents and conditions from the following table, listing them in the order of use. 1 sodium ethoxide
in ethanol 2 ethanol +
acid catalyst & heat 3 H3O(+)
heat 4 CO2 then
H3O(+) 5 Mg in ether
6 PBr3 7 NaBH4 in alcohol 8 CH2I2 in ether
Zn(Cu) 9 BrCH2CO2C2H5 10 (CH3CO)2O
+ pyridine
A) 1 then 9 then 3 then 2
B) 7 then 6 then 5 then 10 then 2
C) 3 then 7 then 6 then 5 then 10 then 2
D) 8 then 3 then 2
--------------------------------------------------------------------------------
59 A C7H9N base reacts with sodium nitrite and hydrochloric acid at 0 ºC, giving a clear solution.
On heating with KCN and Cu2(CN)2 a gas evolves, and continued heating with conc. HCl yields a C8H8O2 crystalline acid.
Heating this acid with aqueous KMnO4 produces a C8H6O4 product, which dehydrates on strong heating to give a crystalline C8H4O3 compound.
What is the C7H9N base?
A) benzylamine
B) N-methylaniline
C) para-toluidine (the toluidines are aminotoluenes)
D) ortho-toluidine
--------------------------------------------------------------------------------
60 Heating diethyl malonate with 2 equivalents of ethyl acrylate and 1.2 equivalents of sodium ethoxide,
followed by neutralization of the base, produces a C15H22O7 compound.
Heating this compound in 10%H4SO4 yields a C7H10O3 crystalline carboxylic acid.
What is this product?
A) 2-oxocyclohexane-1-carboxylic acid
B) 3-oxocyclohexane-1-carboxylic acid
C) 4-oxocyclohexane-1-carboxylic acid
D) 3-(2-oxocyclobutyl)propanoic acid
42 How would the bond strength of the C:C double bond in an alkene compare to that of a C:C single bond in the corresponding alkane?
A) The double bond would have the same strength as the single bond.
B) The double bond would be stronger than, but less than twice as strong as the single bond.
C) The double bond would have exactly twice the strength of the single bond.
D) The double bond would have more than twice the strength of the single bond.
--------------------------------------------------------------------------------
43 Which of the following cations is most stable?
A) FH2+
B) OH3+
C) NH4+
D) CH5+
--------------------------------------------------------------------------------
44 The toxic environmental pollutant dioxin is 44.8% carbon, 1.25% hydrogen and 44.0% chlorine. Its molecular weight is 320±10
What is the molecular formula of dioxin?
A) C10H8O4Cl4
B) C12H6O2Cl4
C) C6H2OCl2
D) C12H4O2Cl4
--------------------------------------------------------------------------------
45 Which of the following covalent compounds does not have any formally charged atoms?
A) (CH3)3NO
B) CH2=N=N
C) CH3-O-N=O
D) CH3C≡NO
--------------------------------------------------------------------------------
46 Which of the following statements is not generally correct?
A) endothermic reactions have larger energies of activation than exothermic reactions
B) the rate of a reaction is proportional to its activation energy
C) the rate of a reaction generally increases as the temperature is raised
D) all reactions in which bonds are broken and formed have a significant activation energy
--------------------------------------------------------------------------------
47 Which of the following compounds has no isomer?
A) CH3CH2CH2Cl
B) CH3CHO
C) CH2=CHCl
D) ClCH2CH2Cl
--------------------------------------------------------------------------------
48 Which of the following statements about carbon is not correct?
A) carbon forms strong covalent bonds to itself, allowing chains and rings to be made.
B) carbon expands its valence shell to accomodate more than eight electrons and thus forms double and triple bonds.
C) carbon forms strong covalent bonds to elements like nitrogen and oxygen because it does not have lone pairs of valence electrons to destabilize the bonds.
D) carbon and hydrogen have similar electronegativity and form strong bonds to each other, thus avoiding the high reactivity shown by metal hydrides
25 Which of the following molecular formulas is reasonable for a stable compound?
A) C8H14O2Cl
B) C6H14Br2
C) C7H10NF
D) C30H54N2Cl
--------------------------------------------------------------------------------
26 What formal charges are present in the molecule C6H5C≡N-O?
( all heavy atoms have a valence shell octet, and C6H5- is a phenyl group)
A) N is -1 and C is +1
B) N is +1 and C is -1
C) O is -1 and C is +1
D) O is -1 and N is +1
--------------------------------------------------------------------------------
27 Which statement about members of a homologous series is true ?
A) they are all constitutional isomers.
B) they are always hydrocarbons.
C) each differs from its nearest neighbors by 14 amu.
D) they may also be classified as tautomers.
--------------------------------------------------------------------------------
28 How many structurally distinct (different) sets of hydrogens are present in (CH3)3CCH2OCH3?
A) 2
B) 3
C) 4
D) 8
--------------------------------------------------------------------------------
29 The pKas of H2CO3 are 6.4 & 10.3. The pKa of HOBr is 8.7. If equimolar amounts of Na2CO3 and HOBr are dissolved in water what will be the predominant anionic species in the resulting solution?
A) HCO3-1 and BrO-1
B) CO3-2, Br-1 and OH-1
C) HCO3-1 and Br-1
D) CO3-2 and 2 BrO-1
--------------------------------------------------------------------------------
30 Which of the following is a conjugate base of CH3NHOH?
A) CH3NH-1
B) CH3NHO-1
C) CH3NH3+1
D) CH2=NOH-1
21 Which of the following compounds has a C-H bond with the lowest bond dissociation energy?
A) C2H6
B) C6H6
C) C2H2
D) CH3CH=CH2
--------------------------------------------------------------------------------
22 In order for a reagent to behave as a nucleophile it must have ...
A) an overall positive charge.
B) an overall negative charge.
C) a non-bonding electron pair.
D) a nitrogen or sulfur atom.
--------------------------------------------------------------------------------
23 The H–C–O bond angle in H2C=O (formaldehyde) is approximately .....
A) 90º
B) 109º.
C) 120º.
D) 180º.
12 Which of the following compounds would be considered an electrophilic reagent?
A) NH3
B) Br2
C) CH3OH
D) NH2OH
--------------------------------------------------------------------------------
13 Which of the following compounds would you expect to be most soluble in water?
A) CH2Cl2
B) C6H12 (cyclohexane)
C) CH3CH2OH
D) C2H5OC2H5
--------------------------------------------------------------------------------
14 The following compounds have similar molecular weights. Which has the highest boiling point?
A) CH3CH=O
B) C2H5OH
C) CH3OCH3
D) CH3CH2CH3
--------------------------------------------------------------------------------
15 Which of the following compound pairs are constitutional isomers?
A) CH3CH2OCH3 and CH3CH2CHO
B) CH3CH2CHO and CH3CH2CH2OH
C) CH3COCH2CH3 and CH3CH2COCH3
D) CH3CH2CH2CHO and CH3COCH2CH3
--------------------------------------------------------------------------------
16 Which of the following compounds may be classed as a protic solvent?
A) tert-butanol
B) diethyl ether
C) n-hexane
D) acetone
4 Which of the following molecules will not have a dipole moment?
A) CH3Cl
B) CH3OCH3
C) CH2Cl2
D) CCl4
--------------------------------------------------------------------------------
5 Which of the following molecules has a dipole moment?
A) CBr4
B) CH2=CH2
C) BF3
D) SO2
--------------------------------------------------------------------------------
6 Which of the following molecules has a linear shape?
A) NH3
B) H2S
C) CO2
D) H2CO
--------------------------------------------------------------------------------
7 Which of the following intermediates is pyramidal in shape?
A) H3C(+)
B) H2C:
C) H3C:(-)
D) HC≡C:(-)
--------------------------------------------------------------------------------
8 In which compound does carbon have the highest oxidation state?
A) CH4
B) HCN
C) H2CO
D) CH2Cl2
--------------------------------------------------------------------------------
9 Which of the following statements applies to a C10H14O2 compound?
A) it may have 2 double bonds and 2 rings
B) it may have 3 double bonds and 0 rings
C) it may have 1 triple bond and 3 rings
D) it may have 0 double bonds and 3 rings
Which of the following is a correct name for (CH3)2C=CHCOCH3?
A) 2-methyl-2-penten-4-one
B) 4-methyl-3-penten-2-one
C) 1,3-dimethyl-2-pentenal
D) isopentenone
--------------------------------------------------------------------------------
2. Which of the following is 3,3-diphenylpropanal?
A) C6H5CH2CH(C6H5)CHO
B) C6H5CH2CH2COC6H5
C) (C6H5)2CHCH2CHO
D) (C6H5)2CHCH2COC6H5
--------------------------------------------------------------------------------
3. Which of the following compounds is not named correctly? A) 2-methyl-3-heptanone (CH3)2CHCOCH2CH2CH2CH3
B) phenylacetaldehyde C6H5CH2CHO
C) 4-hexyn-2-one CH3COCH2C≡CCH3
D) para-bromoacetophenone p-BrC6H4CH2COCH3
7 Which of the following reactions is a good method for preparing an aldehyde?
A) Jones' reagent and a 1º-alcohol
B) Jones' reagent and a 2º-alcohol
C) PCC and a 1º-alcohol
D) H2SO4 a 1º-alcohol and heat
9 Which of the following statements is not generally true?
A) C=O is stronger than an equivalent C=C
B) C=O has a larger bond dipole than C=C
C) aldehydes and ketones have higher boiling points than similarly sized alkenes
D) alkenes add nucleophiles more rapidly than aldehydes or ketones of similar structure
--------------------------------------------------------------------------------
10 Which of the following compounds exchanges the largest number of hydrogens for deuterium when treated with KOD in D2O?
A) 3-methyl-1,2-cycloheptanedione
B) 2-methyl-1,3-cycloheptanedione
C) 5-methyl-1,3-cycloheptanedione
D) 6-methyl-1,4-cycloheptanedione
--------------------------------------------------------------------------------
11 Four C8H14O ketones are examined by 13Cnmr spectroscopy.
One of them has five distinct carbon signals. Which of the following fits this fact?
A) 4,4-dimethylcyclohexanone
B) 3,3-dimethylcyclohexanone
C) 2,2-dimethylcyclohexanone
D) 2,2,4,4-tetramethylcyclobutanone
--------------------------------------------------------------------------------
12 Which of the following compounds could not be converted to pentanal in one or two steps?
A) 1-pentyne
B) trans-5-decene
C) 2-pentanone
D) 1-pentanol
--------------------------------------------------------------------------------
13 Treatment of cyclohexanone with an excess of H218O produces 18O labeled cyclohexanone.
Which of the following is a likely intermediate in this isotope exchange? (the isotope is not named)
A) 1-cyclohexen-1-ol
B) 1,1-cyclohexanediol
C) 2-cyclohexen-1-one
D) 1,2-cyclohexanediol
--------------------------------------------------------------------------------
14 Reaction of C6H5CHBr2 with NaOH in aqueous THF is likely to produce which product?
A) C6H5CHBrOH
B) C6H5CH(OH)2
C) C6H5CHO
D) C6H5CO2H
--------------------------------------------------------------------------------
15 Which of the following carbonyl compounds reacts most rapidly with nucleophilic reagents?
A) benzaldehyde
B) 3,3-dimethylbutanal
C) acetophenone
D) 2,2-dimethylcyclohexanone
--------------------------------------------------------------------------------
16 Which of the following amines would be best chosen for preparing an enamine derivative from cyclohexanone?
A) dimethylamine
B) ethylamine
C) trimethylamine
D) hydroxylamine
--------------------------------------------------------------------------------
17 Which reaction or sequence of reactions would best accomplish the following synthesis?
A) CH3NH2, acid catalyst & heat
B) CH2=NH, acid catalyst & heat
C) (i) NH3 acid catalyst & heat; (ii) CH2I2 & Zn(Cu)
D) (i) HCN & NaCN; (ii) LiAlH4 in ether
--------------------------------------------------------------------------------
18 Heating cyclopentanone with either: I ethyl amine, or II diethylamine, together with an acid catalyst leads to different results.
Which of the following best describes this difference?
A) I gives an imine & II fails to react
B) I gives an enamine & II fails to react
C) I gives an imine & II gives an enamine
D) I gives an enamine & II gives an imine
--------------------------------------------------------------------------------
19 Which reaction or sequence of reactions would be best used to convert cyclohexanone to cis-1,2-cyclohexanediol?
A) PCC in CH2Cl2 and base
B) (i) NaBH4; (ii) H3PO4 & heat; (iii) OsO4 in pyridine
C) (i) NaBH4; (ii) H3PO4 & heat; (iii) C6H5CO3H
D) (i) NaBH4; (ii) OsO4 in pyridine
23 Which of the following reaction sequences would be best for converting cyclohexanol to methylenecyclohexane, (CH2)5C=CH2 ?
A) (i) H3PO4 & heat; (ii) CH2I2 + Zn(Cu)
B) (i) PCC in CH2Cl2; (ii) CH3MgBr; (iii) H3PO4 & heat
C) PCC in CH2Cl2; (ii) (C6H5)3P=CH2
D) CH2N2 & heat
--------------------------------------------------------------------------------
24 Which of the following Wittig reagents would be useful for converting R2C=O into R2CHCH=O?
A) (C6H5)3P=CH-OCH3
B) (C6H5)3P=CH-CH3
C) (C6H5)3P=Cl2
D) (C6H5)3P=CH-CH=CH2
--------------------------------------------------------------------------------
25 Two equivalents of the Wittig reagent (CH3)2C=CH-CH=P(C6H5)3 were allowed to react with a C4H4O2 compound.
The chief product was 2,11-dimethyl-2,4,6,8,10-dodecapentaene, (CH3)2C=CH(CH=CH)3CH=C(CH3)2.
What was the C4H4O2 compound used in this reaction?
A) 2-butyne-1,4-diol
B) 1,2-cyclobutanedione
C) 1,3-butadien-2,3-diol
D) 2-butenedial
--------------------------------------------------------------------------------
26 Which of the following reactions would not be a useful way of preparing 1-phenyl-2-butanol?
A) phenylacetaldehyde + ethylmagnesium bromide
B) butanal + phenylmagnesium bromine
C) propanal + benzylmagnesium bromine
D) 1-phenyl-2-butanone + NaBH4
--------------------------------------------------------------------------------
27 Which of the following reactions would not be a useful way of preparing 2-phenyl-2-butanol?
A) 2-butanone + phenylmagnesium bromine
B) acetophenone + ethylmagnesium bromide
C) cis-2,3-dimethyloxirane + phenylmagnesium bromide
D) ethylphenylketone + methylmagnesium iodide
--------------------------------------------------------------------------------
28 In the reaction of (R)-3-phenyl-2-butanone with methylmagnesium iodide, what happens to the configuration of the stereogenic center?
A) nothing, it remains unchanged
B) inversion takes place
C) racemization occurs
D) the product is achiral
--------------------------------------------------------------------------------
29 Which of the following reactions would not be useful for converting 4,4-diethylcyclohexanone into 1,1-diethylcyclohexane?
A) Wolff-Kishner reduction (N2H4, strong base & heat)
B) Clemmensen reduction (Zn/Hg, acid & heat)
C) thioacetal reduction (i HSCH2CH2SH & BF3; ii H2 + Raney Ni)
D) LiAlH4 in THF & heat
--------------------------------------------------------------------------------
30 Which of the following is a semicarbazone derivative of an aldehyde (RCHO)?
A) RCH=N-NHCONH2
B) RCH=N-OH
C) RCH=N-NH2
D) RCH=N-C(CH3)3
--------------------------------------------------------------------------------
31 Which of the following isomers is most acidic ?
A) 3,4-hexanedione
B) 2,5-hexanedione
C) 2,4-hexanedione
D) hexanedial
--------------------------------------------------------------------------------
32 You have two C6H10O ketones, I and II. Both are optically active, but I is racemized by treatment with base and II is not.
Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula C6H12.
What reasonable structures may be assigned to I and II?
A) I is 3-methyl-4-penten-2-one; II is 4-methyl-1-penten-3-one
B) I is 2-methylcyclopentanone; II is 3-methylcyclopentanone
C) I is 3-methylcyclopentanone; II is 2-methylcyclopentanone
D) I is 2-ethylcyclobutanone; II is 3-ethylcyclobutanone
--------------------------------------------------------------------------------
33 Jones' reagent oxidizes aldehydes to carboxylic acids but normally does not oxidize ketones.
What intermediate species is most likely responsible for the aldehyde oxidation?
A) a carbonyl hydrate
B) an enol tautomer
C) an oxonium conjugate acid of the aldehyde
D) an acylium cation
35 A C5H12O compound is optically active, and is oxidized by PCC in CH2Cl2 to an optically active C5H1OO product, which is racemized in acid or base.
Which of the following best fits these facts?
A) 2-pentanol
B) 2-methoxybutane
C) 2-methyl-1-butanol
D) 3-methyl-1-butanol
--------------------------------------------------------------------------------
36 Which of the following aldehydes, used alone, will undergo an aldol reaction?
A) formaldehyde, CH2O
B) butanal, CH3(CH2)2CHO
C) benzaldehyde, C6H5CHO
D) 2-propenal, CH2=CHCHO
--------------------------------------------------------------------------------
37 Which of the following reaction sequences would be best for preparing 2,2-dimethyl-3-hexanone from butanal?
A) (i) addition of tert-butylmagnesium bromide in ether; (ii) hydrolysis workup; (iii) PCC in CH2Cl2
B) (i) NaBH4; (ii) PBr3; (iii) Mg in ether; (iv) 2,2-dimethylpropanal, followed by hydrolysis
C) (i) Wittig reaction with (C6H5)3P=C(CH3)2; (ii) BH3 in THF; (iii) H2O2 & base; (iv) PCC in CH2Cl2
D) (i) Wittig reaction with (C6H5)3P=CHC(CH3)3; (ii) BH3 in THF; (iii) H2O2 & base; (iv) PCC in CH2Cl2
--------------------------------------------------------------------------------
38 How can 1,1,1,2,2-pentadeutero-3,3-dimethylpentane, C2H5C(CH3)2C2D5, be prepared from 3,3-dimethyl-2-pentanone?
A) (i) NaOD in D2O (excess); (ii) LiAlD4 in ether; (iii) D2O workup
B) (i) Wolff-Kishner reduction with N2D4 in ROD; (ii) NaOD in D2O (excess)
C) (i) Zn & DCl in CH3CO2D; (ii) LiAlD4 in ether; (iii) D2O workup
D) (i) NaOD in D2O (excess); (ii) HSCH2CH2SH + BF3; (iii) D2 + Raney Ni catalyst
--------------------------------------------------------------------------------
39 Acetaldehyde reacts with (R)-1,2-propanediol (acid catalyst) to give two isomeric acetals. What is their isomeric relationship?
A) they are constitutional isomers
B) 2 they are diastereomers
C) 3 they are enantiomers
D) they are conformers
42 Base induced elimination of 3-chlorocyclohexanone I is much faster than elimination of cyclohexyl chloride II.
What is the major factor accounting for this difference?
A) the halogen in I is less hindered
B) the halogen in I is forced to be axial
C) elimination of I produces a more stable conjugated double bond
D) I rapidly forms an enolate anion, and this immediately eliminates a stable chloride ion
--------------------------------------------------------------------------------
43 Which of the following optically active compounds will be racemized on treatment with warm alcoholic sodium ethoxide?
(R)-2,2,6-trimethylcyclohexanone (R)-2,2,5-trimethylcyclohexanone (R)-2-methyl-2-phenylcyclohexanone (R)-4-methyl-2-cyclohexen-1-one
I II III IV
A) I and II
B) I and III
C) I and IV
D) all compounds will be racemized
--------------------------------------------------------------------------------
44 A C5H8 hydrocarbon is reacted with BH3 in THF, followed by oxidation with alkaline hydrogen peroxide.
Treatment of the resulting product with PCC in CH2Cl2 produces a chiral ketone, formula C5H8O.
What hydrocarbon best fits these facts?
A) 1-methylcyclobutene
B) methylenecyclobutane
C) vinylcyclopropane
D) cyclopentene
--------------------------------------------------------------------------------
45 Which of the following compounds would not be a possible product from the mixed aldol reaction of acetaldehyde and butanal?
A) 3-hydroxybutanal
B) 2-ethyl-3-hydroxybutanal
C) 3-ethyl-2-hydroxyhexanal
D) 3-hydroxyhexanal
--------------------------------------------------------------------------------
46 A C7H12O2 compound gives a positive Tollens' silver mirror test and a positive iodoform test.
Which of the following would satisfy these facts?
A) 2-hydroxy-3,3-dimethylcyclopentanone
B) 2,5-heptanedione
C) 2,2-dimethyl-3-oxopentanal
D) 2,2-dimethyl-4-oxopentanal
--------------------------------------------------------------------------------
47 The iodoform test for methyl ketones is not widely used anymore.
Which of the following spectroscopic tools is best for providing equivalent information?
A) UV-Visible
B) 1H nmr
C) 13C nmr
D) infrared
--------------------------------------------------------------------------------
48 An aldol condensation is used to prepare 1,3-diphenyl-2-propenone, C6H5CH=CHCOC6H5.
Which combination of reactants will lead to this product?
A) enolate donor=acetaldehyde; carbonyl acceptor=benzaldehyde
B) enolate donor=phenylacetaldehyde; carbonyl acceptor=phenylacetaldehyde
C) enolate donor=acetophenone; carbonyl acceptor=benzaldehyde
D) enolate donor=propiophenone; carbonyl acceptor=benzaldehyde
53 2,2-Dimethyl-1,3-propanediol is coveniently prepared by heating 2-methylpropanal with excess formaldehyde and Ca(OH)2.
What sequence of reactions takes place in this synthesis?
A) dehydrogenation to 2-methyl-2-propenal followed by addition of formaldehyde
B) dehydrogenation to dimethylketene followed by addition of formaldehyde
C) a crossed aldol reaction followed by a crossed Cannizzaro reaction
D) a crossed Cannizzaro reaction followed by a crossed aldol reaction
6 Which of the following is a proper name for (CH3)2CHCH2NHCH2CH2CH(CH3)2?
A) 2,7-dimethyl-4-azaoctane
B) butylpentylamine
C) 2,7-dimethylpropylbutylamine
D) 3-amino-2,7-dimethyloctane
--------------------------------------------------------------------------------
7 Ethylmethylamine cannot be resolved under normal conditions. Why?
A) the favored configuration is not chiral.
B) it isomerizes rapidly with the achiral isomer trimethylamine.
C) the nitrogen atom rapidly inverts its configuration leading to a racemic mixture.
D) the C–N bond is not stable under conditions used for resolution