20 Benzylamine may be alkylated as shown in the following equation.
C6H5CH2NH2 + R-X ----> C6H5CH2NHR (pyridine is used to scavange the HX produced here)
Which of the following alkyl halides is best suited for this reaction?
A) (CH3)3CCH2Br
B) C6H5Br
C) C6H5CH2Br
D) (CH3)3CCl
I KEPt THE NAME OF THE QUESTION AS SUCH BECAUSE I WILL KEEP ON POSTING MY QUESTIONS IN THIS TAG ONLYYYYYYYY I WILL ADD NEW QUESTIONS DAILYYYYYYYYYYYY whats more than organic in chemistry.. so i will start with that
1]Convert aniline to phenyl acetic acid in NOT MORE THAN 5 steps....
2)Convert cyclopent2ene1one to cyclobutanol in NOT MORE THAN 5 steps....
3]give the steps for the mechanism for the conversion of cyclohexanone oxime in the presence of H+, heat to caprolactam
4]convert benzaldehyde to 3-phenylpropan-1-ol
5]what is fugisity
-
UP 0 DOWN 0 9 213

213 Answers
11 Dibutylamine, (C4H9)2NH, and anisole, C6H5OCH3, have similar boiling points, and are relatively insoluble in water.
How might a mixture of these compounds be separated into the pure components?
A) i) dissolve mixture in ether; ii) extract the anisole into 10% aqueous NaOH
B) i) dissolve mixture in ether; ii) extract the amine into 10% aqueous HCl
C) i) dissolve mixture in ether; ii) extract the amine into 10% aqueous NaOH
D) i) dissolve mixture in ether; ii) extract the anisole into 10% aqueous HCl
22 Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
A) H2 (excess) & Pt catalyst
B) LiAlH4 in ether
C) Fe in 15% HCl
D) Zn in 10% HCl
--------------------------------------------------------------------------------
23 What reagent is the source of nitrogen in the Gabriel synthesis of amines?
A) sodium azide, NaN3
B) sodium nitrite, NaNO2
C) potassium cyanide, KCN
D) potassium phthalimide, C6H4(CO)2NK
--------------------------------------------------------------------------------
24 In order to prepare a 1º-amine incorporating an additional CH2 group from an alkyl halide, what reagent is often used as the nitrogen source?
A) sodium amide, NaNH2
B) sodium azide, NaN3
C) potassium cyanide, KCN
D) potassium phthalimide, C6H4(CO)2NK
--------------------------------------------------------------------------------
25 What set of conditions would be useful for preparing a 2º-amine?
A) 2º-RBr + NaNH2
B) (i) 2º-RBr + NaN3 (ii) H2 & Pt
C) (i) 1º-RNH2 + 1º-RCHO (ii) H2 & Pt
D) (i) 2 1º-RBr + potassium phthalimide (ii) H3O+ & heat
--------------------------------------------------------------------------------
26 What reagent is used in the Hinsberg test of amines?
A) (CH3CO)2O & pyridine
B) C6H5SO2Cl in aq. NaOH
C) NaNO2 in aq. H2SO4
D) CH3I (excess) followed by AgOH
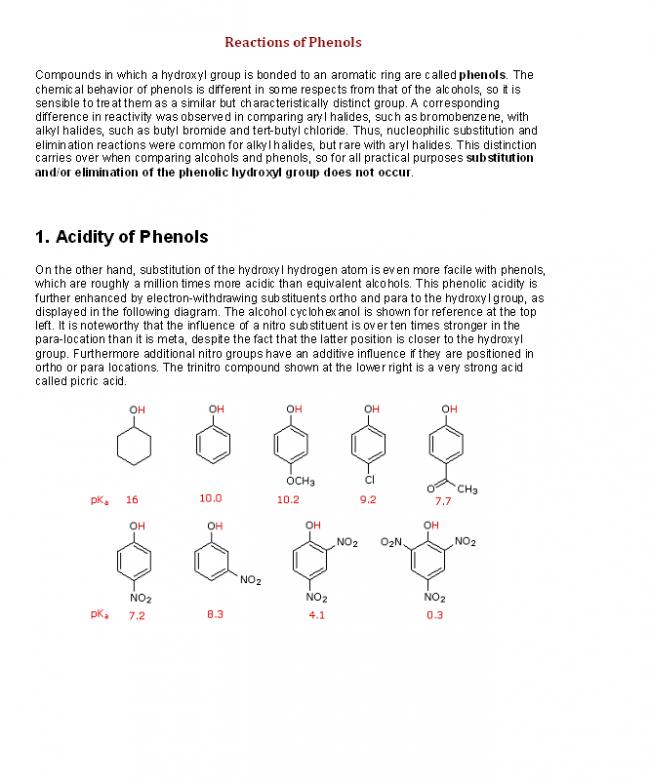
34 Many kinds of amino alcohols are known, but α-amino alcohols such as 1-dimethylaminocyclopentanol are not stable under most conditions.
Which of the following statements best accounts for their instability?
A) carbon does not like to form bonds to two more electronegative atoms such as O and N.
B) the α-isomer rapidly rearranges to the more stable β amino alcohol.
C) rapid loss of water (a stable small molecule) leads to an imine product.
D) rapid loss of amine leads to a stable carbonyl compound (aldehyde or ketone).
--------------------------------------------------------------------------------
35 Which of the following amines reacts most rapidly with para-nitrophenylacetate, p-NO2C6H4OCOCH3?
A) para-methoxyaniline,.p-CH3OC6H4NH2
B) para-nitroaniline, p-NO2C6H4NH2.
C) aniline, C6H5NH2.
D) cyclopentylamine, C5H9NH2.
--------------------------------------------------------------------------------
36 What product mixture is expected from reaction of (S)-2-aminobutane with 2-butanone in the presence of NaBH3CN?
A) a pair of enantiomers.
B) a pair of diastereomers, both meso.
C) a pair of diastereomers, one meso one chiral.
D) a pair of diastereomers, both chiral (not enantiomers).
--------------------------------------------------------------------------------
37 Which of the following compounds is the strongest base?
A) (CH3)2NCH2CO2CH3.
B) (CH3)2NCOCH3.
C) (CH3)2NC≡N.
D) (CH3)2N-N=O.
41 A nitrogen containing compound dissolves in 10% aq. sulfuric acid..
The Hinsberg test (C6H5SO2Cl in base) gives a solid product that is not soluble in 10% aq. NaOH..
Which of the following would best fit these facts?
A) N,N-dimethylaniline, C6H5N(CH3)2.
B) N-methylbenzamide, C6H5CONHCH3.
C) N-methylaniline, C6H5NHCH3.
D) benzylamine, C6H5CH2NH2.
--------------------------------------------------------------------------------
42 Which of the following reagents would be best for converting phenylacetamide (C6H5CH2CONH2) to benzylamine (C6H5CH2NH2)?
A) LiAlH4 in ether.
B) i) P2O5 & heat; ii) LiAlH4 in ether.
C) H2 & Pt catalyst.
D) aqueous NaOBr.
--------------------------------------------------------------------------------
43 The Hinsberg test of a C5H14N2 compound produces a solid that is insoluble in 10% aq. NaOH.
This solid derivative dissolves in 10% aq. sufuric acid. Which of the following would best fit these facts?
A) NH2CH2CH2CH2N(CH3)2.
B) (CH3)2NCH2CH2NHCH3.
C) NH2CH2C(CH3)2CH2NH2.
D) (CH3)2NCH2N(CH3)2.
--------------------------------------------------------------------------------
44 Which of the following reagents and conditions would be best for the preparation of cyclohexylamine?
A) cyclohexanone + NH3 + NaBH3CN.
B) cyclohexylbromide + 2 NH3.
C) cyclohexylbromide + NaNH2.
D) cyclohexene + NH3.
--------------------------------------------------------------------------------
45 Which of the following reagents would be best for converting phenylacetamide (C6H5CH2CONH2) to phenethylamine (C6H5CH2CH2NH2)?
A) H2 & Pt catalyst.
B) NaBH3CN.
C) LiAlH4 in ether.
D) aqueous NaOBr.
49 Which of the following statements concerning amine oxides is not true?
A) chiral amine oxides may be resolved.
B) the nitrogen has a positive formal charge.
C) the nitrogen hybridization is sp3.
D) all types of amines can form amine oxide derivatives.
--------------------------------------------------------------------------------
50 Amines are well known to be stronger bases and nucleophiles than alkenes.
Why do enamines, such as 1-dimethylaminocyclopentene, preferentially react with electrophiles at a double bond carbon rather than at nitrogen?
A) the nitrogen is sterically hindered by alkyl substituents.
B) nitrogen is more electronegative than carbon.
C) the carbocation formed by electrophilic attack at C-2 is stabilized by pi-bonding with the lone pair of electrons on nitrogen.
D) ammonium cations are less stable than carbocations.
--------------------------------------------------------------------------------
51 A C5H13N compound gives a base soluble derivative in the Hinsberg test (C6H5SO2Cl in base).
The 13Cnmr spectrum of this compound has four resonance signals at δ 8.7, 29.9, 37.5 & 49.5 ppm.
Which of the following fits these facts best?
A) 1,1-dimethylpropylamine.
B) isopropyldimethylamine.
C) 2,2-dimethylpropylamine.
D) N-methyl-2-methylpropylamine.
--------------------------------------------------------------------------------
52 Reaction of para-chloroaniline with acetic anhydride in pyridine gave a mixture of 94% of para-chloroacetanilide, contaminated with 6% unreacted amine.
Which of the following treatments would be best used to purify the amide?
A) react the unreacted amine with methyl iodide.
B) wash an ether solution of the crude product with concentrated brine (aq. NaCl).
C) wash an ether solution of the crude product with 5% aqueous sulfuric acid.
D) wash an ether solution of the crude product with 5% aqueous sodium carbonate.
4 Which of the following is a chiral C5H12O 1º-alcohol?
A) 3-methyl-2-butanol
B) 2-methyl-2-butanol
C) 3-methyl-1-butanol
D) 2-methyl-1-butanol
--------------------------------------------------------------------------------
5 Which of the following reagents would you expect to react with bromocyclopentane by an SN2 mechanism?
A) C2H5OH
B) C2H5O(-) K(+)
C) NaCN
D) (CH3)3N
--------------------------------------------------------------------------------
6 Chloroethane, C2H5Cl, does not react with methanol under mild conditions.
What reagent could be added to the reaction mixture to increase the rate of substitution.?
A) HCl (conc.)
B) NaOH
C) NH4OH
D) AgNO3
--------------------------------------------------------------------------------
7 Which of the following compounds is unlikely to react with sodium metal?
A) C2H5OC2H5
B) C2H5OH
C) C2H5Br
D) C2H5NH2
--------------------------------------------------------------------------------
8 The reaction of sodium ethoxide with iodoethane to form diethyl ether is classified as ...
A) an electrophilic substitution
B) a nucleophilic substitution
C) a radical substitution
D) an electrophilic addition
--------------------------------------------------------------------------------
9 Compound X reacts with HI. The product of this reaction, when treated with KOH in ethanol, gives Y ( an isomer of X ).
Ozonolysis of Y (H2O2 workup) produces two compounds: a two carbon carboxylic acid, and a four carbon ketone.
What is X?
A) 2-methyl-2-pentene
B) 4-methyl-1-pentene
C) 2,3-dimethyl-2-butene
D) 3-methyl-1-pentene
--------------------------------------------------------------------------------
10 The SN2 reaction of 1-chloro-3-methylbutane with sodium methoxide is relatively slow, but can be accelerated by the addition of a small amount of NaI.
How is this catalysis best explained?
A) The sodium cation helps pull off the chloride anion
B) The iodide anion activates the methoxide nucleophile
C) SN2 reaction of iodide ion converts the alkyl chloride to the more reactive alkyl iodide
D) The NaI changes the mechanism to SN1
--------------------------------------------------------------------------------
11 Which one of the following alcohols will be oxidized by Jones' reagent (CrO3 in 50% sulphuric acid) to a ketone having the same number of carbon atoms ?
A) 1-methylcyclohexanol
B) 3,3-dimethylcyclopentanol
C) 3-methyl-1-hexanol
D) 3-ethyl-3-hexanol
--------------------------------------------------------------------------------
12 What reagent would be suitable for distiguishing 1-methoxy-3-methyl-2-butene from its isomer 4-methyl-3-penten-1-ol?
A) bromine in methylene chloride
B) KMnO4 in aqueous base
C) AgNO3 in dilute NH4OH
D) sodium metal suspended in hexane
--------------------------------------------------------------------------------
13 Synthesis of hexane-3,4-diol from trans-3-hexene may be accomplished in two ways:
(i) OsO4 hydroxylation & (ii) C6H5CO3H epoxidation followed by NaOH opening of the epoxide ring.
Which of the following statements about the products from these reactions is correct?
A) the two methods giive the same product
B) (i) gives a chiral isomer, (ii) gives an achiral isomer
C) (i) gives an achiral isomer, (ii) gives a chiral isomer
D) two different isomers are formed, but both are chiral
--------------------------------------------------------------------------------
14 Reaction of 1,4-dibromobutane with Mg turnings in ether gives the bis-Grignard reagent, BrMgCH2CH2CH2CH2MgBr.
What is the product from the reaction of meso-2,3-dibromobutane with Mg under the same conditions?
A) trans-2-butene
B) cis-2-butene
C) meso-CH3CH(MgBr)CH(MgBr)CH3
D) racemic-CH3CH(MgBr)CH(MgBr)CH3
18
I OH(-) II CH3CO2(-) III HO2(-) IV H2O
The above molecules and ions are all nucleophiles. What is the relative order of their reactivity in an SN2 reaction with ethyl bromide?
A) I > II > III > IV
B) IV> III > II > I
C) III > I > II > IV
D) II > III > IV > I
--------------------------------------------------------------------------------
19 In the SN2 reaction of cyanide ion with (CH3)2CHCH2CH2X what is the relative order of reactivity for the following X substituents?
I X = F II X = Cl III X = Br IV X = I
A) I > II > III > IV
B) IV> III > II > I
C) III > I > II > IV
D) II > III > IV > I
--------------------------------------------------------------------------------
20 Which of the following does not convert a 1º-hydroxyl group into a good leaving group for a SN2 reaction?
A) SOCl2
B) CH3SO2Cl
C) PBr3
D) NaI
--------------------------------------------------------------------------------
21 How is the following reaction best classified?
(CH3)3CBr + (CH3CH2)3N -----> (CH3)2C=CH2 + (CH3CH2)3NH(+) Br(-)
A) SN2 substitution
B) E2 elimination
C) electrophilic addition
D) cationic rearrangement
--------------------------------------------------------------------------------
22 Which of the following isomeric chlorides will undergo SN2 substitution most resdily?
A) 4-chloro-1-butene
B) 1-chloro-1-butene (cis or trans)
C) 1-chloro-2-butene (cis or trans)
D) 2-chloro-1-butene
--------------------------------------------------------------------------------
23 Which reagent would be best for achieving an E2 elimination of 3-chloropentane?
A) C2H5ONa
B) CH3CO2Na
C) NaHCO3
D) NaI
--------------------------------------------------------------------------------
24 Which reaction conditions would be best for the synthesis of isobutyl sec-butyl ether
CH3CH2CH(CH3)-O-CH2CH(CH3)2 ?
A) (CH3)2CHCH2OH + H2SO4 + heat
B) CH3CH2CH(CH3)OH + H2SO4 + heat
C) CH3CH2CH(CH3)ONa + (CH3)2CHCH2Br
D) (CH3)2CHCH2ONa + CH3CH2CH(CH3)Br
26 A C7H13Br compound reacts with KOH in ethanol to form 3-methylcyclohexene as the major product.
What is a likely structure for the starting alkyl bromide?
A) cis-4-methylcyclohexyl bromide
B) trans-3-methylcyclohexyl bromide
C) cis-2-methylcyclohexyl bromide
D) trans-2-methylcyclohexyl bromide
--------------------------------------------------------------------------------
27 A synthesis of 2,5-dimethyl-2-hexanol from 2-methylpropene requires the formation of two four-carbon intermediates, X and Y.
These intermediates combine to give the desired product after the usual hydrolysis work-up.
Select appropriate methods of preparing X and Y from 2-methylpropene
A) X add HBr, then react with Mg in ether Y add water, acid-catalysis
B) X add HBr (peroxides), then react with Mg in ether Y react with C6H5CO3H in CH2Cl2
C) X add HOBr Y add B2H6in ether, then NaOH
D) X add HOBr Y add HBr (peroxides) ,then react with Mg in ether
--------------------------------------------------------------------------------
28 All of the following alkyl bromides react by SN2 substitution when treated with sodium cyanide in methanol.
Which one does not undergo an inversion of configuration?
A) (R)-1-bromo-2-methylbutane
B) (S)-2-bromo-3-methylbutane
C) (R)-1-bromo-3,3-dimethylcyclohexane
D) cis-4-ethyl-1-bromocyclohexane
hey b555 why du u discuss something beyond syllabus....
does that help?
(like claisen etc)
does it improve understanding?
Allene Chemistry
The simplest cumulated diene is 1,2-propadiene, CH2=C=CH2, also known as allene. Indeed, cumulated dienes are often called allenes. The central carbon in such compounds is sp-hybridized (it has only two bonding partners), and the double bond array is linear as a result. Since the π-bonds of allenes are orthogonal, the planes defined by the end carbon substituents are also orthogonal. As shown in the following diagram, the overall configuration of allenes resembles that of an elongated tetrahedron. An interesting consequence of this configuration is that allenes having two different substituents on each of the terminal carbon atoms are chiral.
More than two double bonds may have a cumulated structure, as we find in 1,2,3-butatriene (CH2=C=C=CH2) and 1,2,3,4-pentatetraene (CH2=C=C=C=CH2). The carbon atoms in such cumulenes all have a linear configuration, but the configuration of the terminal substituents depends on the number of cumulated double bonds. For an even number of double bonds, an orthogonal configuration of terminal substituents (as in allene) will be observed. For an odd number of double bonds, the terminal substituents and all the carbons between them will lie in a plane. If the terminal substituents at each end are different, the even double bond compounds will have enantiomeric stereoisomers; whereas, the odd double bond compounds will exist as cis-trans diastereoisomers.
Some instructive physical properties of a simple cumulated diene, 1,2-butadiene, compared with its conjugated diene and alkyne isomers are presented in the following table. From the heats of hydrogenation we see that the methylallene is thermodynamically the least stable of these isomers, with the conjugated diene being most stable. The ionization potential is intermediate between the alkyne and the conjugated diene (note than an electron volt is equivalent to 23.05 kcal/mol), suggesting that the pi-electrons in the allene are less strongly bound than in the alkyne. Finally, the gas phase basicity or proton affinity is close to that of the conjugated diene, and slightly greater than that of the alkyne
Addition Reactions of Allenes
Allenes undergo the usual electrophilic addition reactions, and one of the double bonds may even serve as a dienophile in a Diels-Alder reaction. However, the regioselectivity of electrophilic addition may seem surprising when examined with reference to the generally accepted order of cation stability.
Carbocation
Stability CH3(+) ≈ RCH=CH(+) < RCH2(+) ≈ RCH=CR(+) < R2CH(+) ≈ CH2=CH-CH2(+) < C6H5CH2(+) ≈ R3C(+)
Methyl 1º-Vinyl 1º 2º-Vinyl 2º 1º-Allyl 1º-Benzyl 3º
Thus, addition of HBr to allene gives 2-bromopropene not 3-bromobropene (allyl bromide). From the relative stability of vinyl and allyl cations the latter product would be expected.
CH2=C=CH2 + H–Br CH3CBr=CH2 not BrCH2CH=CH2
allene 2-bromopropene allyl bromide
To understand why the reaction path proceeding by way of an allyl cation is not favored here we must recall the orthogonal orientation of the two pi-electron systems. As shown in the following diagram, protonation of the center, sp-hybridized, carbon atom generates an allyl-like carbocation, but the empty p-orbital of this cation (red) is initially oriented 90º to the π-orbital (blue) of the adjacent double bond, so no conjugation can occur. In order to aquire the stabilization and charge delocalization expected for an allyl cation, a 90º rotation about the bond joining the carbocation to the double bond must take place. Since this can only occur after the carbocation is fully formed, the transition state for central carbon protonation has the high activation energy associated with any 1º-carbocation formation. Indeed, the inductive effect of the adjacent double bond probably raises the transition state energy even further. Consequently, formation of a 2º-vinyl cation by protonation at an end carbon (bottom equation) is kinetically favored.
Some other addition reactions to allenes are shown in the following equations. The first example demonstrates that bromine adds readily to one of the allene double bonds. The inductive effect of the halogens retards addition of a second equivalent of bromine, but this may take place under more forcing conditions. The oxymercuration example results in nucleophile (water) bonding to a terminal carbon, probably because SN2 opening of the cyclic mercurinium intermediate is favored at that site. The last example is interesting because it shows that independent stabilization of a terminal carbocation, e.g. by benzyl resonance, changes the initial site of electrophilic attack to the central (sp-hybridized) carbon. The resulting stabilized cation may then achieve further stabilization by rotating to conjugate with the remaining double bond. The two isomeric addition products shown herecome from nucleophile bonding at both ends of the resulting allyl cation intermediate.
Addition reactions of isolated dienes proceed more or less as expected from the behavior of simple alkenes. Thus, if one molar equivalent of 1,5-hexadiene is treated with one equivalent of bromine a mixture of 5,6-dibromo-1-hexene, 1,2,5,6-tetrabromohexane and unreacted diene is obtained, with the dibromo compound being the major product (about 50%).
CH2=CH(CH2)2CH=CH2 + Br2 BrCH2CHBr(CH2)2CH=CH2 + BrCH2CHBr(CH2)2CHBrCH2Br + CH2=CH(CH2)2CH=CH2
5,6-dibromo-1-hexene 1,2,5,6-tetrabromohexane 1,5-hexadiene
Similar reactions of conjugated dienes, on the other hand, often give unexpected products. The addition of bromine to 1,3-butadiene is an example. As shown below, a roughly 50:50 mixture of 3,4-dibromo-1-butene (the expected product) and 1,4-dibromo-2-butene (chiefly the E-isomer) is obtained. The latter compound is remarkable in that the remaining double bond is found in a location where there was no double bond in the reactant. This interesting relocation requires an explanation.
CH2=CH-CH=CH2 + Br2 BrCH2CHBr-CH=CH2 + BrCH2CH=CHCH2Br
3,4-dibromo-1-butene 1,4-dibromo-2-butene
The expected addition product from reactions of this kind is the result of 1,2-addition, i.e. bonding to the adjacent carbons of a double bond. The unexpected product comes from 1,4-addition, i.e. bonding at the terminal carbon atoms of a conjugated diene with a shift of the remaining double bond to the 2,3-location. These numbers refer to the four carbons of the conjugated diene and are not IUPAC nomenclature numbers. Product compositions are often temperature dependent, as the addition of HBr to 1,3-butadiene demonstrates.
CH2=CH-CH=CH2 + HBr
reaction temperature CH3CHBr-CH=CH2 +
1,2 addition yield CH3CH=CHCH2Br
1,4 addition yield
0 ºC
40 ºC 70%
15% 30%
85%
Bonding of an electrophilic atom or group to one of the end carbon atoms of a conjugated diene (#1) generates an allyl cation intermediate. Such cations are stabilized by charge delocalization, and it is this delocalization that accounts for the 1,4-addition product produced in such addition reactions. As shown in the diagram, the positive charge is distributed over carbons #2 and #4 so it is at these sites that the nucleophilic component bonds. Note that resonance stabilization of the allyl cation is greater than comparable stabilization of 1,3-butadiene, because charge is delocalized in the former, but created and separated in the latter.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
The Diels-Alder reaction is an important and widely used method for making six-membered rings, as shown on the right. The reactants used in such reactions are a conjugated diene, simply referred to as the diene, and a double or triple bond coreactant called the dienophile, because it combines with (has an affinity for) the diene. The Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
The Diels-Alder reaction is a single step process, so the diene component must adopt a cis-like conformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile. Such conformations are called s-cis, the s referring to the single bond connecting the two double bonds. The s-cis and s-trans conformers of 1,3-butadiene are shown in the preceding diagram. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are generally in rapid equilibrium, permitting the use of all but the most hindered dienes as reactants in Diels-Alder reactions. In its usual form, the diene component is electron rich, and the best dienophiles are electron poor due to electron withdrawing substituents such as CN, C=O & NO2. The initial bonding interaction reflects this electron imbalance, with the two new sigma-bonds being formed simultaneously, but not necessarily at equal rates.
Stereospecificity
We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. Likewise, if the terminal carbons of the diene bear substituents, their relative configuration will be retained in the adduct. Using the earlier terminology, we could say that bonding to both the diene and the dienophile is syn. An alternative description, however, refers to the planar nature of both reactants and terms the bonding in each case to be suprafacial (i.e. to or from the same face of each plane). This stereospecificity also confirms the synchronous nature of the 1,4-bonding that takes place.
. Which of the following is true for 3-methylbutanal?
a) This compound may be classified as an aldehyde.
b) This compound may be classified as a ketone.
c) An aldol reaction takes place on treatment with NaOH solution.
d) There is no reaction with LiAlH4 in ether solution.
e) An excess of CH3MgBr in ether reacts to give 4-methyl-2-pentanol.
f) Wolff-Kishner reduction gives butane.
g) This compound is an isomer of 3-pentanone.
--------------------------------------------------------------------------------
2. Which of the following is true for 3-methyl-2-butanone?
a) It may be prepared by CrO3 oxidation of 2-methyl-2-butanol.
b) Reaction with NaBH4 gives a secondary alcohol.
c) It may be prepared by acidic Hg2+ catalyzed hydration of 3-methyl-1-butyne.
d) It forms a silver mirror on treatment with Ag(NH3)2.
e) This compound is an isomer of 4-penten-1-ol.
--------------------------------------------------------------------------------
3. Which of these methods would serve to prepare 1-phenyl-2-propanol?
a) Addition of benzyl Grignard reagent to acetaldehyde (ethanal).
b) Addition of phenyl lithium to propylene oxide (methyloxirane).
c) Addition of phenyl Grignard reagent to acetone (2-propanone).
d) Acid-catalyzed hydration (addition of water to) of 2-phenyl-1-propene.
e) Addition of methyl Grignard reagent to acetophenone (methyl phenyl ketone).
f) Addition of methyl Grignard reagent to phenylacetaldehyde.
4. Oxidation Reactions of Alcohols
Simple 1º and 2º-alcohols in the gaseous state lose hydrogen when exposed to a hot copper surface. This catalytic dehydrogenation reaction produces aldehydes (as shown below) and ketones, and since the carbon atom bonded to the oxygen is oxidized, such alcohol to carbonyl conversions are generally referred to as oxidation reactions. Gas phase dehydrogenations of this kind are important in chemical manufacturing, but see little use in the research laboratory. Instead, alcohol oxidations are carried out in solution, using reactions in which the hydroxyl hydrogen is replaced by an atom or group that is readily eliminated together with the alpha-hydrogen. The decomposition of 1º and 2º-alkyl hypochlorites, referred to earlier, is an example of such a reaction.
RCH2–OH + hot Cu RCH=O + H2
RCH2–O–Cl + base RCH=O + H–Cl
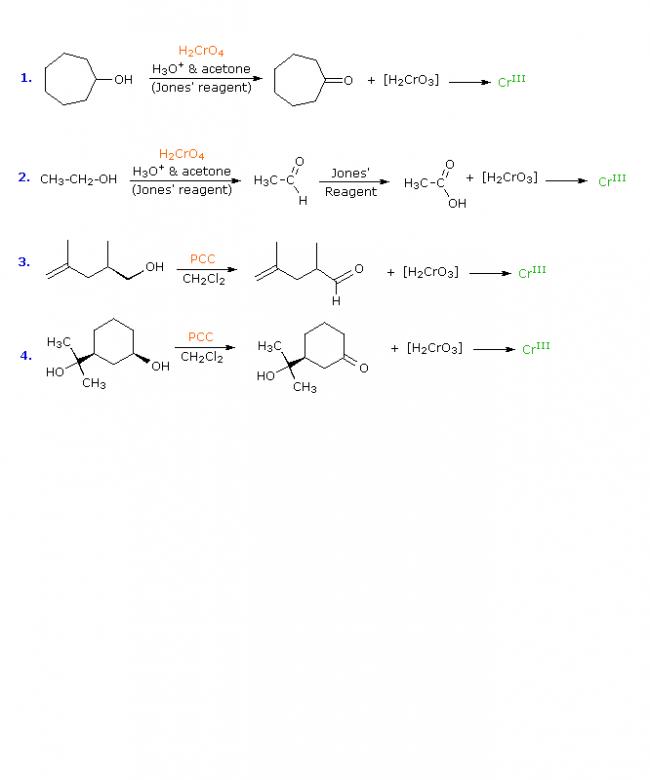
The most generally useful reagents for oxidizing 1º and 2º-alcohols are chromic acid derivatives. Two such oxidants are Jones reagent (a solution of sodium dichromate in aqueous sulfuric acid) and pyridinium chlorochromate, C5H5NH(+)CrO3Cl(–), commonly named by the acronym PCC and used in methylene chloride solution. In each case a chromate ester of the alcohol substrate is believed to be an intermediate, which undergoes an E2-like elimination to the carbonyl product. The oxidation state of carbon increases by 2, while the chromium decreases by 3 (it is reduced). Since chromate reagents are a dark orange-red color (VI oxidation state) and chromium III compounds are normally green, the progress of these oxidations is easily observed. Indeed, this is the chemical transformation on which the Breathalizer test is based. The following equations illustrate some oxidations of alcohols, using the two reagents defined here. Both reagents effect the oxidation of 2º-alcohols to ketones, but the outcome of 1º-alcohol oxidations is different. Oxidation with the PCC reagent converts 1º-alcohols to aldehydes; whereas Jones reagent continues the oxidation to the carboxylic acid product, as shown in the second reaction. Reaction mechanisms for these transformations are displayed on clicking the "Show Mechanism" button. For the first two reactions the mechanism diagram also shows the oxidation states of carbon (blue Arabic numbers) and chromium (Roman numbers). The general base (B:) used in these mechanisms may be anything from water to pyridine, depending on the specific reaction.
Two structural requirements for the oxidation to carbonyl products should now be obvious:
1. The carbon atom bonded to oxygen must also bear a hydrogen atom.
Tertiary alcohols (R3C–OH) cannot be oxidized in this fashion.
2. The oxygen atom must be bonded to a hydrogen atom so that a chromate ester intermediate (or other suitable leaving group) may be formed.
Ethers (R–O–R) cannot be oxidized in this fashion.
The fourth reaction above illustrates the failure of 3º-alcohols to undergo oxidation. The second reaction mechanism explains why 1º-alcohols undergo further oxidation by Jones reagent. The aqueous solvent system used with this reagent permits hydration (addition of water) to the aldehyde carbonyl group. The resulting hydrate (structure shown below the aldehyde) meets both the requirements stated above, and is further oxidized by the same chromate ester mechanism. Water is not present when the PCC reagent is used, so the oxidation stops at the aldehyde stage.
Another chromate oxidizing agent, similar to PCC, is pyridinium dichromate, (C5H5NH(+) )2 Cr2O7(–2), known by the acronym PDC. Both PCC and PDC are orange crystalline solids that are soluble in many organic solvents. Since PDC is less acidic than PCC it is often used to oxidize alcohols that may be sensitive to acids. In methylene chloride solution, PDC oxidizes 1º- and 2º-alcohols in roughly the same fashion as PCC, but much more slowly. However, in DMF solution saturated 1º-alcohols are oxidized to carboxylic acids. In both solvents allylic alcohols are oxidized efficiently to conjugated enals and enones respectively
Consider the possibility of the following reaction:
2-propanol
+
H3PO4 (conc.) and heat (>150 °C)
?
If you believe a reaction will take place, draw the structural formula for the chief organic product using the drawing window on the right. If no reaction is expected, draw the starting alcohol structure.
Consider the possibility of the following reaction:
cyclopentanol
+
PBr3 & heat
Consider the possibility of the following reaction:
2-methyl-1-propanol
+
C6H5CO2H, acid catalyst and heat
?
Consider the possibility of the following reaction:
cyclohexanol
+
Na (elemental metal)
Consider the possibility of the following reaction:
2-methyl-2-propanol
+
hydrazine (N2H4) & heat
Consider the possibility of the following reaction:
2-methyl-1-propanol
+
PBr3 & heat
Solve questions
Q.1. Two samples of HI each of 5 gm were taken separately in two vessel of volume 5& 10 ltr respectively at . The extent of dissociation of HI will be
1)more in 5 ltr vessel
2)more in 10 ltr vessel
3)equal in both vessel
4)NIL at both
Q.2) For the reaction the equilibrium constant is most likely to be changed by
1)The addition of acetic acid soln.
2) The addition of suitable catalyst.
3)The addition of acetate ion.
4) Heating the given mixture.
Q.3. At constant temp. , the equilibrium constant Kp for the decomposition of the reaction is expressed by
where P=pressure & x=extent of decomposition . Which of the following statement is true ?
1) Kp increases with increase of P.
2) Kp increase with increase of x.
3) Kp increase with decrease of x.
4)Kp remain constant with change in P & x.
Q.6.
Statement 1: Alkenes can give electrophilic as well as nucleophilic addition reactions.
and
Statement 2: Addition of HCl to CH2 = CH - Cl will mainly produce CH3 - CHCl2.
1. Statement 1 is True, statement 2 is True; statement 2 is a correct explanation for statement 1.
2. Statement 1 is True, statement 2 is True; statement 2 is not a correct explanation for statement 1.
3. Statement 1 is True, statement 2 is False.
4. Statement 1 is False, statement 2 is True.
Q.7. KMnO4 and MnO2 react in alkaline medium (KOH) to give K2MnO4. What is n factor of K2MnO4 in the reaction?
1. 3
2. 2
3. 2/3
4. 3/2
q1)two moles of an ideal gas are expand isothermally and irreversibly frm 1litre to 10litre at 300k.the enthalpy change in kj for the process is?
q2)the total energyin cal of one mole of an ideal monoatomic gas at 300k is ?
q3)for a 3dxy,, 3dy^2 electron ,the orbital angular momentum is?