is there any pbm with the question
y no one is repling
give the condn A and B for which the reaction would follow the reaction as in fig
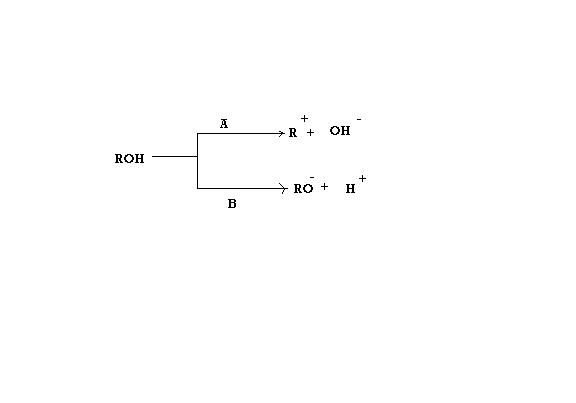
case I :the alcohol is primary
caseII :the alcohol is secondary
caseIII :the alcohol is teritary
-
UP 0 DOWN 0 1 4

4 Answers
voldy
·2009-01-18 01:46:29
case 1 )
B; use a base .
A ; use conc . H2SO4.
case 2) same as above.
case 3) same I thnk. can't thik of any now. :P
voldy
·2009-01-18 01:55:26
B : a base obviously abstratcs the most acidic proton .
A : here protonation takes place , then H2O leaves as it's a good leavnig grp.