2 > 3 > 1 > 4?
2 would be greatest, followed by 3 for presence of alkyl group, and then 1 which has just nitrogen's lone pair, and the aldehyde group sucks electrons from the ring.
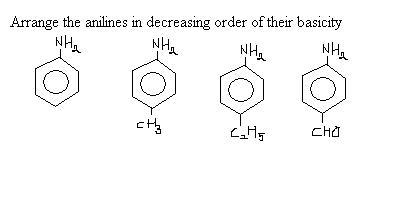
Explain the order for the methyl aniline and ethyl aniline..
The rest are obvious
-
UP 0 DOWN 0 0 5

5 Answers
2>3 why??
isnt ethyl group more elecrton releasing than methyl ??
This is more of basics of organic from 11th..so I'm not so well versed in those lol. Wait for other replies.
the main concept here is the density of electron pair on nitrogen atom ! me and et groups ,due to their strong electron donating abilities repel the l.p on nitrogen atom ! what we need to know is that the repulsion due to 1 ethyl group is more than that due to 1methyl group(in fact 2 methyl groups)...hence ,
3>2>1>4
...also, going by the experimentally found pka value , the pka for methyl aniline is 4.85 and that for the ethyl aniline is 5.11 (more the pka , the more is the basicity)