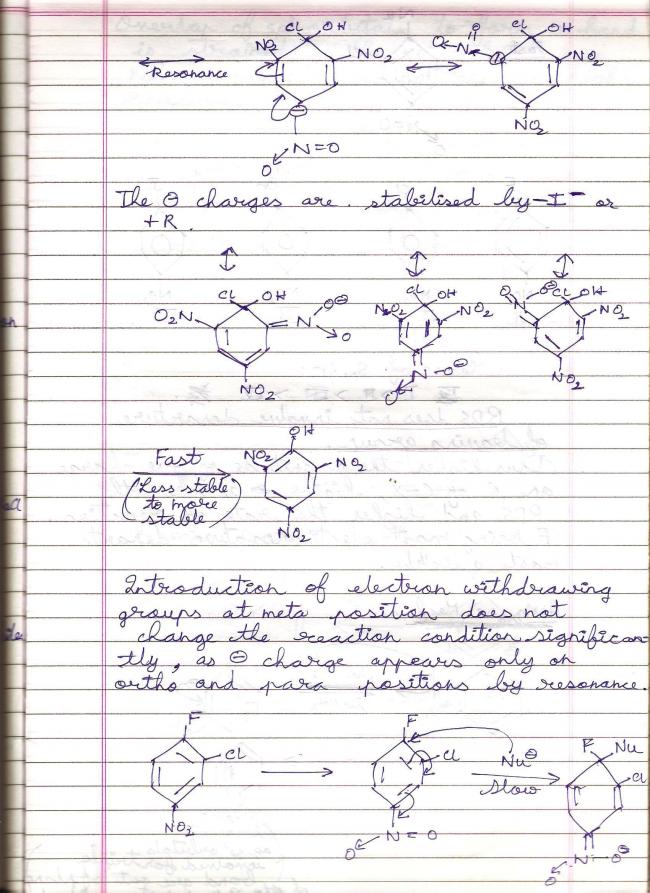
39
39Aryl halides deactivated by EWGs undergo SNAr mechanism because the reaction condition relaxes considerably each time an EWG is added to the benzene molecule. The nucleophile finds it easier to attack when the negative charge density on benzene is reduced.
Substitution by the benzyne mechanism occurs under drastic conditions, specifically in the presence of a strong base and under high temperature. It is 1) The conditions, 2) The substituents attached to benzene which matter in deciding which mechanism is followed.
p-chlorotoluidene follows the benzyne mechanism if given those drastic conditions. It will not undergo SNAr in any case, because that requires a level of deactivation to the ring which groups like -NO2 provide.
 1
1Aryl halides deactivated by EWGs undergo SNAr mechanism because the reaction condition relaxes considerably each time an EWG is added to the benzene molecule. The nucleophile finds it easier to attack when the negative charge density on benzene is reduced.
So you mean deactivation occurs when EWG is present at any position w.r.t halogen?
 1
1Ok I get it, whether -OCH3 is present at ortho,meta,para, the reaction always occurs through benzyne mechanism as -OCH3 has weaker inductive effect which is not able to stabilise the intermediate carbanion in SNar mechanism. In other words, -OCH3 is not that much effective in deactivating the ring.
Correct me if I'm wrong.
and Thanks for posting your notes.
 39
39Almost gotten the hang of it...add this to your understanding - the EWG's inductive strength is only half the battle. Drastic conditions must also be provided in benzyne reactions.
You can get the entire collection of my organic notes from the link in my signature. I've uploaded them on scribd.