11
11swaraj.......
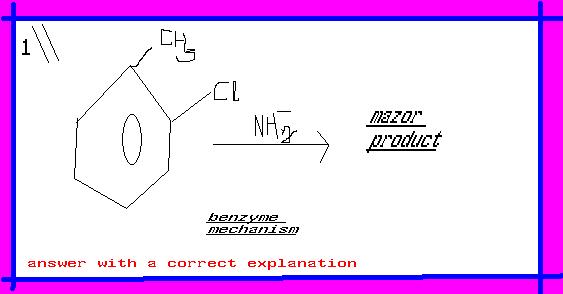
in benzyne reaction u must represent the bonding sequence
ok , product -NH2 SUBSTITUTED AT meta position with respect to methyl group.because when u formed benzyne both cine subs. other direct subs , becoz electron density increase at ortho positon due to methyl hence meta subs. is mazor product. if still u have problem contact me telephonically.
mukesh
 29
29Assertion Reasoning on Benzyne ...
Statement - 1 ..Benzene is aromatic whereas benzyne is anti-aromatic..
Statement - 2..Benzene follows huckel-rule ans has 6Ï€ electrons..whereas Benzyne has 8Ï€ electrons..
 6
6i thnk statement one and two both are correct...
 11
11as in benzyne the pie of triple bond can not b delocalised hence it can not b anti aromatic.i think
 1
1D) Statement 1 is FALSE
Anti-aromatic:
The molecule must have 4n π electrons where n is any integer.
The molecule must be cyclic.
The molecule must have a conjugated pi electron system.
The molecule must be planar.
Benzyne is aromatic the meaning of pi electron in respect of huckels rule..when it say pi electron it means no. of electron participating in conjugation...in case of benzyne, pi bonds are mutually perpendicular and only one pair of electron will participate in conjugation..hence six pi electron in conjugation and huckels rule followed.

Yes benzyne is aromatic..... it has a cyclic, planar, conjugated, 6p electron system.
Note that the second p bond (shown as the blue orbitals in the diagram above) of the triple bond is perpendicular and therefore cannot overlap with the aromatic p system (shown as cyan orbitals). This means that the two electrons associated with this bond are not part of the conjugated system.
Benzyne is very reactive due to the strain of the triple bond due to its incorporation into the six membered ring.
Benzyne has a reactive triple bond undergoing addition reactions, including the Diels-Alder reaction:

 29
29Ans is D..
in benzyne the new π bond formed is sp2 - sp2 hybridised and is perpendicular to the plane containing the initial π bonds...so it is not in conjugation with those 6π electrons....
 1
1Benzyne can be called non-aromatic...
 29
29@Vivek..can u explain how?