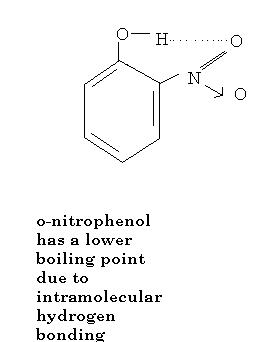
the key is intra and inter molecular hydrogen bonding...what aveek has said is just a part of the answer ! p-nitrophenol has inter whereas o-nitrophenol has intra hydrogen bonds ! boiling involves separation of discrete molecules, which is difficult in the former case !
WHICH OF THE FOLLOWING HAS HIGHER BOILING POINT?
(a)p-nitrophenol (b)o-nitrophenol
give explanation.......i am bit confused
-
UP 0 DOWN 0 0 3

3 Answers
Debotosh..
·2009-12-02 22:19:08