anyone??
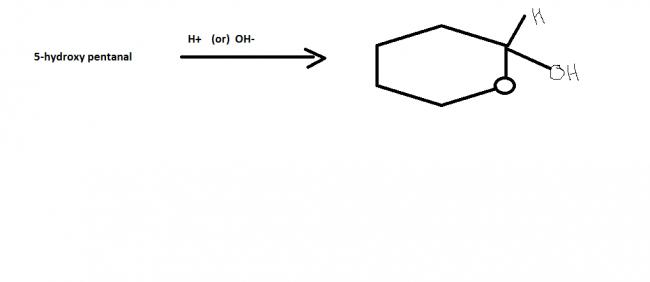 identify incorrect statements for the given reaction,
identify incorrect statements for the given reaction,
1) it's intra molecular ester formation
2)product can be resolved into optically active compounds(BTW, what do we mean by this?pls elaborate)
3) the product formation is not favourable in dil. solutions
4) it is example on nucleophilic addition.
answer: 1 & 3
why is this product not an ester??
pls quick!!
-
UP 0 DOWN 0 0 2

2 Answers
i hvn`t studied carbonyl compounds so i`m answring this purely from the mechanism that i`ve learnt till now....
i think in dil. solutions conc of water is more and water is polar protic solvent....
so the H+ ion will attack the OH-grp and water(which is a good leaving grp) will come out and as result of which a stable carbocation will be formed....since the lone pairs of oxygen will undergo resonance....so the product cannot be formed in dil. solns....this is what i think........but i dnt knw why ester will not be formed