well i don't know the answer...
but when two cycloproply groups are present then it is more stable
than cycloheptan-2,4,6-trieneyl cation ( tropolium ion).
well i don't know the answer...
but when two cycloproply groups are present then it is more stable
than cycloheptan-2,4,6-trieneyl cation ( tropolium ion).
cycloheptan-2,4,6-trieneyl cation
here it is more stable because of the aromaticity of the ring...
also we can call the conjugation warrior to explain stability...
its right that tropolium ion is stable due to aromaticity , but even more
stable than aromatic cation are cyclo propyl methyl carbocations, and if
there are two cyclopropyl group then definitely it will be more stable,
but when only one group is present it is not easy to say....
the main difficulty here is that the reason for stability of cyclo propyl
methyl carbocations is not very clear ( it is becoz of some sort of
interaction b/w bent orbitals and vaccant p orbitals)
don't know more than this:)
sincere advice : even organic chemists dont know the answer properly, hence it is not wise to discuss this question.
Main ideas are:
reason for stability of tropylium cation (aromaticity)
and cyclopropyl methyl cation
comparision is not required (here)
1st is more stable becoz in 1st resonance exist but not in 2nd and also 3 membered ring is unstable
I read about this one in Solomons. The tropylium substrate has an ionisation constant of the order 1030. This observation was explained by the fact that the tropylium cation is extremely stable due to its aromaticity. If you need a solid explanation you will have to look at stuff like orbital overlaps and all...if I find that page in Solomons I'll scan it and put it up here.
@pritish: what abt the second carbocation ?? I think t\it has comparable stability compared totropylium cation
@ashish : Does aromaticity compare to relief of ring strain?
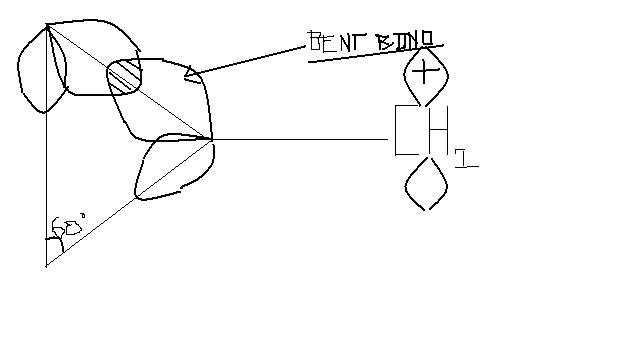
In structure 2 as (horribly) drawn above, in the 3 membered ring, inspite of being sp2 hybridised, the the bond is bent and makes an angle of 60°( instead of 120°).
Hence the p- orbitals remain almost in the same plane as the p orbitals of CH2+.
Thus there is a transfer of elctrons to the vacant p- orbital of CH2+ and hence the structure is highly stable.
In structure 1, the structure is stabilized by aromaticity. but between the 2
strucs, struc1 is more stable(probably) as it is an exception and they wouldnt have given the comparison otherwise!!!
yes scintillating , and that is the reason why the second structure is more stable than benzyl carbocation, (CH3)3C+ ,allylic, etc
Generally Dancing Resonance are more stable than aromatic compounds but only Cylopropylethyl cation is less stable than Tropilium cation..This is an exception