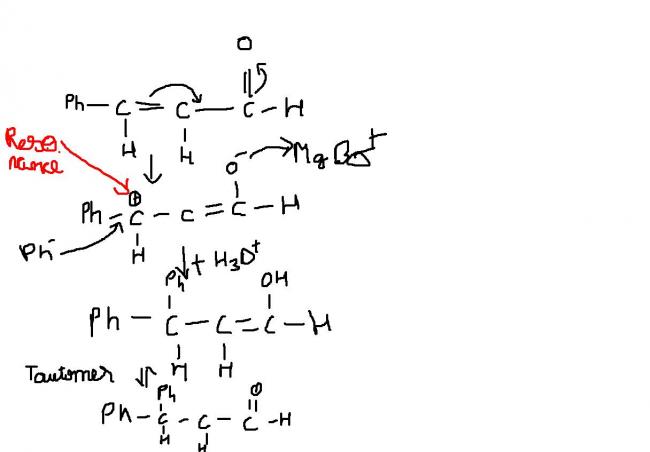With RMgBr, both 1,2 and 1,4 addition products are possible. Steric hindrance decides the major product as the process is irreversible.
The Ph group attached is too bulky for the R- anion to attack the double bonded C attached to it. Hence the major product is a 1,2 addition product, Ph-CH=CH-CH(R)-OMgBr, hydrolysis of which gives an alcohol.
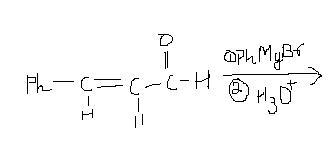
explain the reason why which product will be major 1,2 or 1,4
abhi, please do not answer
this is my doubt
-
UP 0 DOWN 0 0 14

14 Answers
but then wont the alcohol tautomerise to form the keto form?
what if CH3MgBr was attacking
btw our sir said that if it is strong nucleophile/base 1,2 product is major while if it is weak nucleophile/base 1,4 product will be major
Alkanide anions are quite strong bases as their conjugate acids, alkanes, are weak acids. Indeed it would be a 1,2 product for CH3MgBr. Tautomerisation toh baad ki baat hai, you asked about 1,2 or 1,4 product and I answered that...
so, is PhMgBr strong or weak?
yahaan pe tautomerism hoga ya nahiin
btw answer was given as
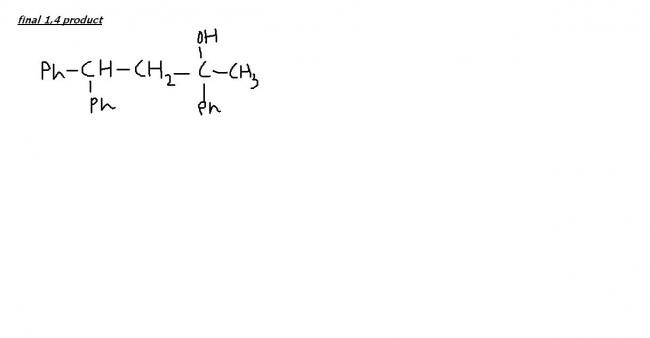
Ph-CH(Ph)-CH2-CH2OH so the doubt arised
Ohhh...its Ph. I didn't notice that! Resonance stabilised anions are weak, obviously. And in your product it cannot tautomerise, there is no double bond which can shift to the oxygen.
Product will be 1,4, sorry about the earlier mistake.
(1) i was talking if the 1,2 product will tautomerise
(2) why product will be 1,4 ? because of resonance??
this is the final product if excess of PhMgBr is there, right??
But then two Phenyl groups would reduce the electrophilicity of the carbonyl carbon wont they?
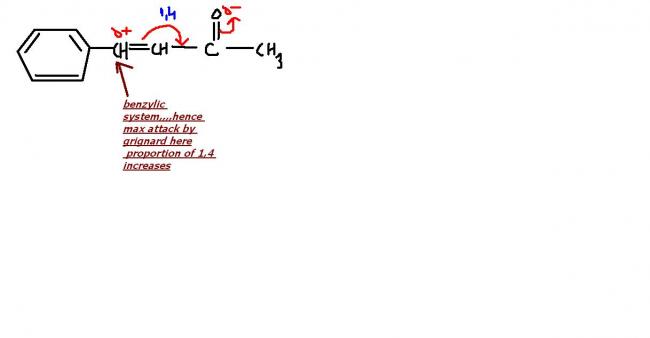
Ph- will be a weak nucleophile n will give majorly 1,4 but here steric hindrance due to bulky phenyl is a issue????
R-Li+ Gives 1,2 and R-Na+ gives 1,4 mostly!!
hey organic ur second part doesnt seem correct to me brother....i think it willl tautomerize back!!
Dekh bhai to decide product for addition of RMgBr, we have decide on basis of steric hindrance only. Ph- is a weak base because it is highly stabilised by resonance. Thus it won't provide strong steric hindrance for a 1,4 product.
And the 1,2 product won't tautomerise because the double bond is not on the alpha,beta carbons of hydroxyl group...though not sure about this, check with others.
You're bringing in many things at once...take it one at a time!