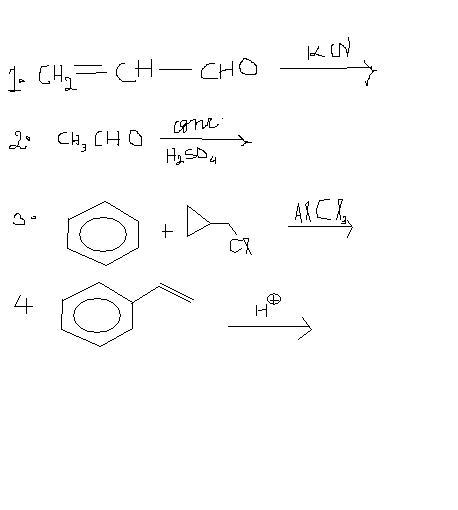
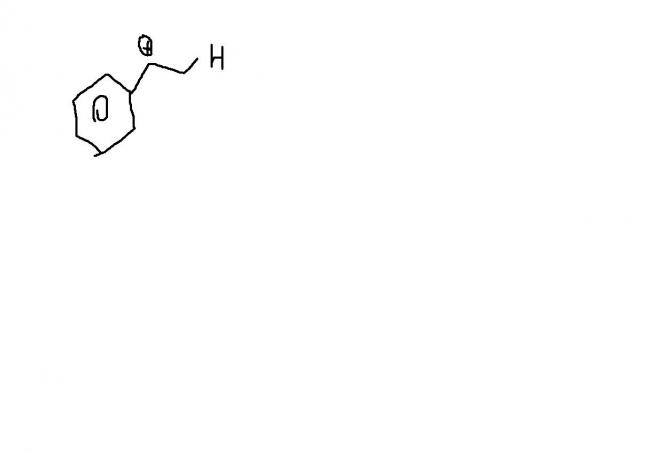
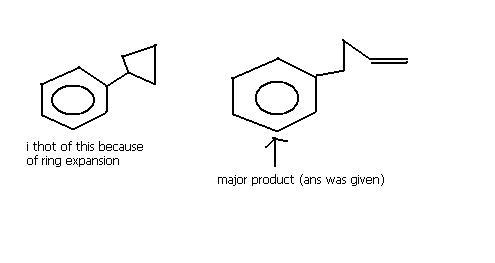
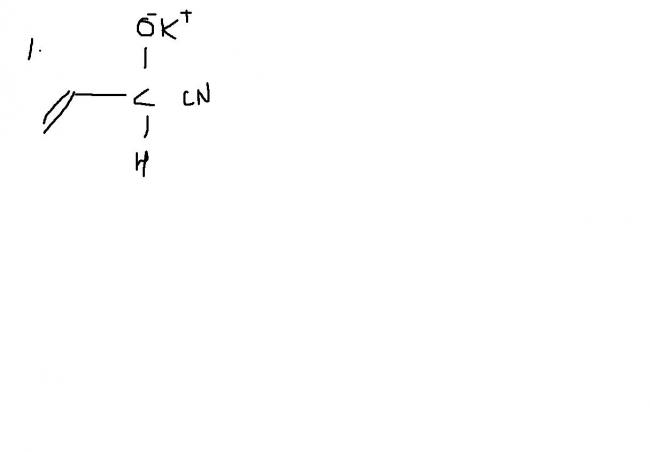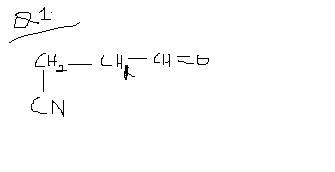hmm i want expert views on this one..
19 Answers
for the third one,we need some idea about DEMJANOV REARRANGEMENT....if you have any idea then please respond in this thread..i will proceed with the best explanation!!!
FOR THE FIRST ONE, the cyanide ion prefers the double bond because the development of the carbanion is easy due to 1,3 conjugation as shown below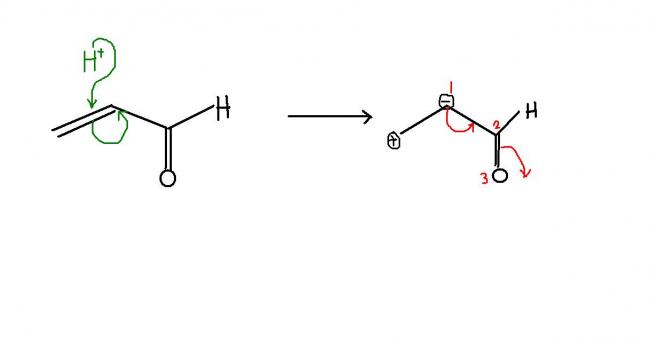
Q1) i guess we have to see the resonance hybrid of the given structure.If we draw the resonance hybrid we can see that the carbon at -C=0 will not be an electrophilic center
For Q2: answer should be paraldehyde. Though there is a doubt regarding that.
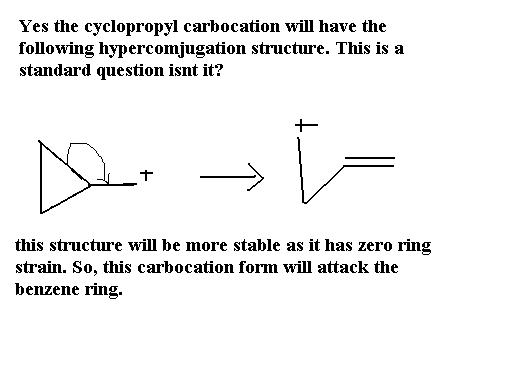
For Q3: ring expansion can't take place, because ring expansion occurs when next structure (after ring expansion) is not strained.. for example: cyclobutane's expansion to cyclopentane 5 membered ring.. which is stable.. so, I think the step given by mahato is correct
for the first question:
the mech. given by debotosh is correct..
the reaction's Michael addition
http://en.wikipedia.org/wiki/Michael_reaction
@organic...
but how can 3rd be Demjanov rearrangement???
I mean Demjanov rearrangement gives rearranged alcohols naa when primary amines and nitrous acid reqact.....
maybe because 3 and four membered rings are highly unstable .............