look in the second figure when u select the longest continuous chain
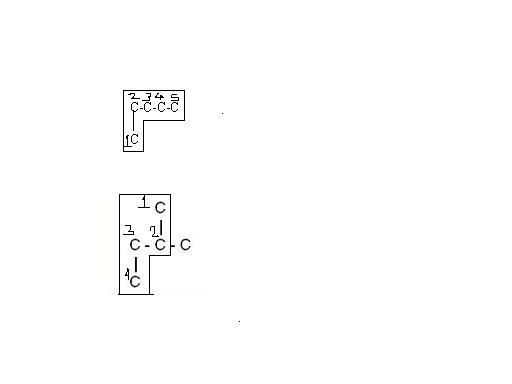
see img 1 it is same an n-pentane
second one is same as 2-methyl pentane
thus fig 1 and 2 are same
fig 3 and fig 5 are same
just the way of looking is different
In the given isomers of pentane why only fig 1, fig 3, and fig 4 will be considered i.e., pentane, 2-methylbutane and 2,2-dimethylpropane why not fig 2 and fig 5 please tell me. I am much confused....??????????????????????
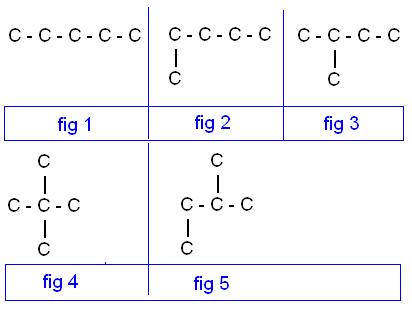
look 5 and 2 have same iupac name if u luk carefully!! thus absolutely same compound!! [1]
Whenever you get the questions where you have to find the structural isomers
(constitutional isomers is also its name) you just do their nomenclature, if the the name is
coming same for some compounds it implies they are the same compound.
look in the second figure when u select the longest continuous chain

see img 1 it is same an n-pentane
second one is same as 2-methyl pentane
thus fig 1 and 2 are same
fig 3 and fig 5 are same
just the way of looking is different