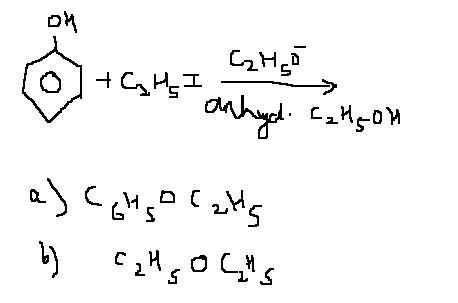probabably......(a) .....as it is a strong base,,,,,,,,,,and a side product wud be (b),,,,,vaise weres pritish ?? :)
16 Answers
It's a strong base as well as strong nucleophile...so there are good chances of substitution through SN2 mechanism also...so answer can be B...
@all.........hey these days it seems all are having fun lollllllll
yaar wats the debate here???????
clearly.....(b) is the right answer[1][for this question,in fact]
these BT people suck!!!!
hey guys, lots of confusion. AISSCE and aieeee are two different guys altogether.
cn we plz hv the reasoning here ? nothing specific but just a simple query !
OOOHHH.......SORRY NEVER KNEW THAT[1]BUT BY THE WAY WAT IS ORIGINAL????
hey , pritish, nothing like dat. we hv the same posters, thats yet. we two are two different guys.
and @ AISSCE , sorry fr the controversial statement, i hv deleted it.
@ eure, i just wanted to know the logic behind the answers given. anyways, here goes my logic.
There are two ways possible :
i) acidic hydrogen from phenol goes to the ethoxide ion and ethanol is formed leaving phenoxide ion as the nucleophile.
as phenoxide ion is though strong , but hindered base hs the possibility of elimination.
ii) ethoxide ion directly attacks the halide. in this case the nucleophile is ethoxide ion , which is strong and non-hindered. thus, the possibility of elimination is negligible.
tus the mechanism goes through 2nd method and diethyl ether is formed.
Since the medium is ethanol so there will be some ethoxide ion left even after extracting the proton from phenol..so in that case there will be competition b/w ethoxide and phenoxide ion..since ethoxide is a better nucleophile so option B will be correct....that's what i think...
@Aieee...just a li'l correction...diethyl ether will be formed as product and not ethyl ester