give aquesous OH-...though chances to react is very very leess
Guys, provide here the easiest and shortest way to convert Chlorobenzene to phenol. Burning Desire answered this once.. but I'm still not able to understand the reason
-
UP 0 DOWN 0 0 12

12 Answers
dow's process...as simple as that !
the process is as follows :
application of aqueous NaOH at 475°C and 200-250 atms pressure !
low reactivity of simple aryl halides toward nucleophilic substitution is illustrated by the observation that temperatures on the order of 350 °C (660 °F) are required in order to convert chlorobenzene to phenol by reaction with sodium hydroxide. Furthermore, the reaction has been shown to proceed by a mechanism different from conventional nucleophilic substitution pathways.
is it that first th addition occurs n den since chlorine is a better leaving group that OH-..so Cl- leaves
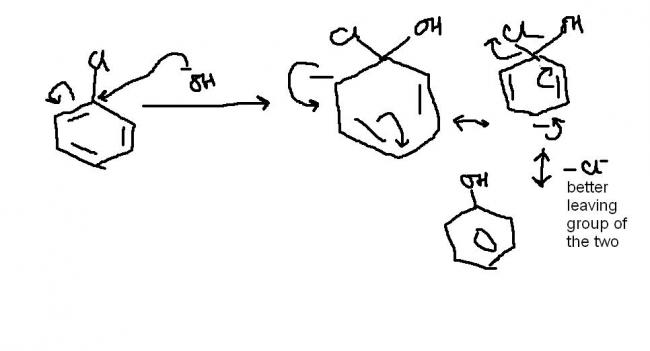
dis is the mechanism...but skul mei itna likhega toh teacher log ko samajh nahi aayega..n they will cut it lolz...
ya two times my teacher told me last year-"beta class me itna zyada dimag mat lagaya karo..."
lolz........
but rohit, SE (electrophilic substitution) should be preferred instead of nucleophilic substituttion reactions.. shouldn't it..
you answr.. I'm getting off now...will read everything later (probably after few hrs)
Add NaOH at 300°C and 200 Atmospheres
C6H5Cl+2NaOH→C6H5ONa+2NaCl+H2O
Now add C6H5ONa+H+/H2O→You GEt Phenol
BUT ALSO THERE IS ONE STEP REACTION
1)C6H5Cl+Steam (catlyst. SiO2) at 425°C→Phenol
You can also use the Dow's process that IS
2) by adding Na2CO3 and H2O