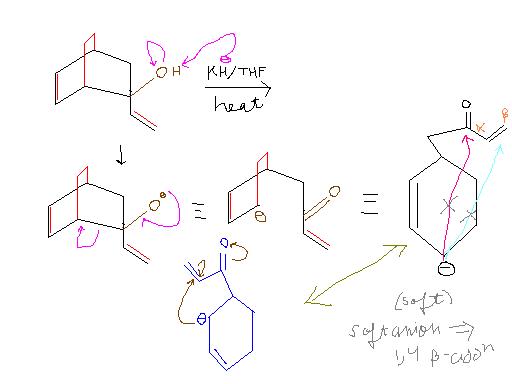
I could find only this about potassium hydride :
http://www.organic-chemistry.org/abstracts/lit1/498.shtm
The major product P of the given reacn is :-
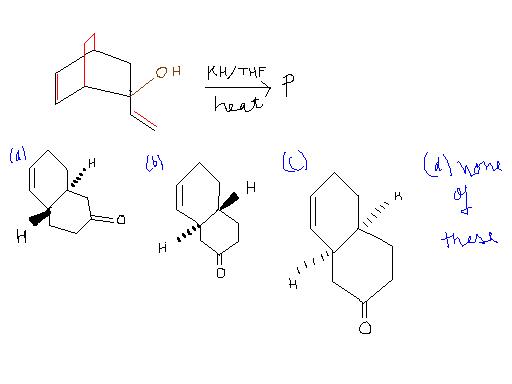
This is not my doubt
-
UP 0 DOWN 0 1 6

6 Answers
Pritish Chakraborty
·2009-11-18 10:32:54
Pritish Chakraborty
·2009-11-18 10:44:01
Okay, KH is a very strong base, so it will abstract a proton. Acidity of alcohols is greater than alkenes, hence it will abstract a proton from -OH. Possibly an internal cyclization after that?