Fr 2) Google helped a bit-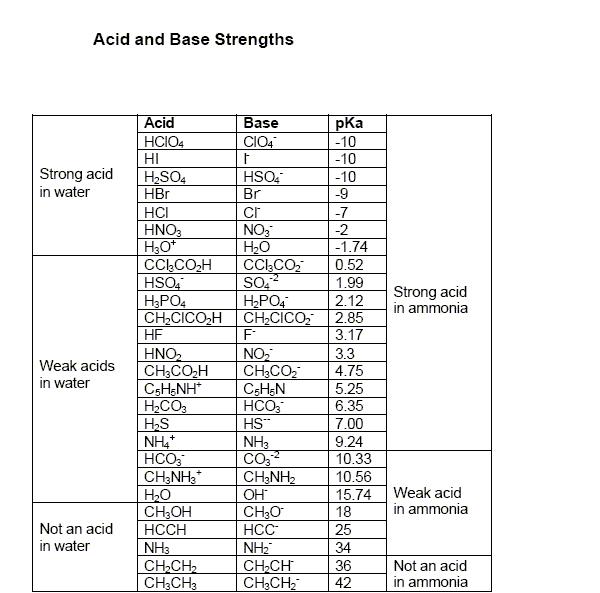

dbts which came to my mind while studying today..
Q1 can conc H3PO4 +conc HNO3 be used as nitrating mixture ??
Q2 we know acetylene is highly acidic...butg it never exists in soln in this form H-C≡C- +H+ ... why ???
Q3 why 2nd step(i.e CH bond breaking step) is rate determining step in sulfonation of benzene though first step has higher activation energy ???
Q1. pKa value of H3PO4 is 2.15 as it is a weak acid. while HNO3 is a strong acid.. In nitration, the nitric acid acts as the base (accepts H+ thereby increasing the rate of formation of the nitronium ion) as it is weaker than H2SO4
But in presence of H3PO4 it will act as an acid i.e. donate H+ to form NO3- ion.. Now there is some amount of common ion effect so conc of NO3- will be reduced..
As NO3- can disproportionate (although quite little) to give NO2+ but due to common ion this will be further reduced to become negligible.
Hence NO
Q3. it is a reversible reaction. But I still think first step is rate determining step. Where did you read that?
thx...
what i read other day was that 2nd step is rate determinin gstep...dont remeber the book name,..becoz read in library