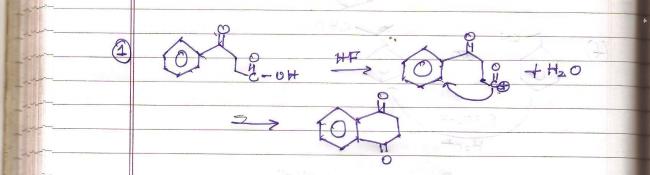
HF is a halogen acid which provides a proton.
Q1 
Q2 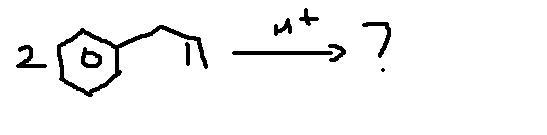
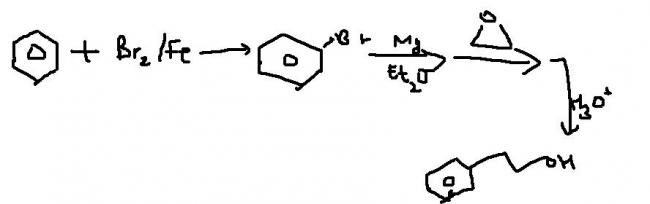
Q3 Synthesise from benzene ..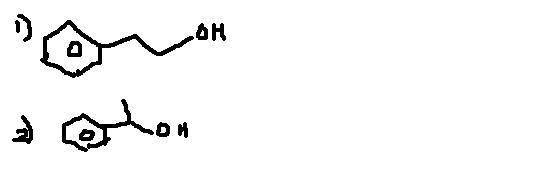
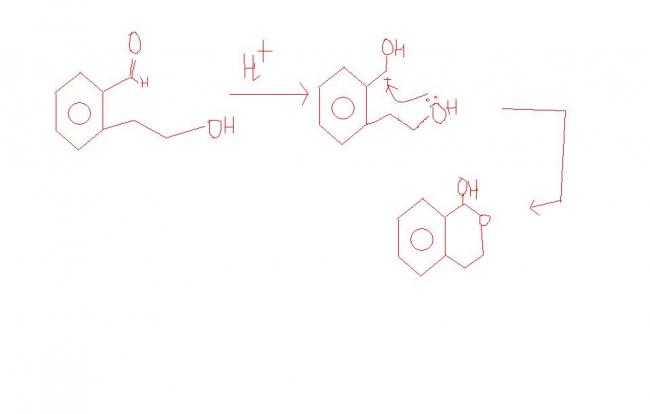
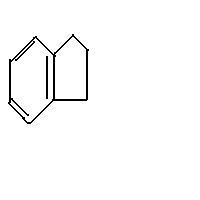
Q4 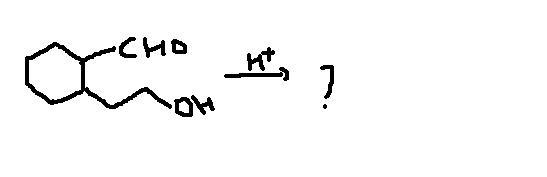
Q5 Identify best reducing agent in step2 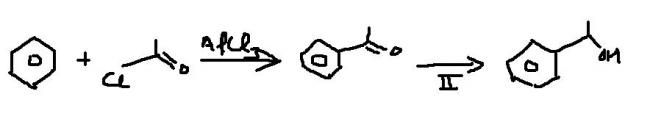
-
UP 0 DOWN 0 1 12

12 Answers
If you want to use Friedel Craft reaction such that no rearrangement takes place, use acylation, and then use Zn/Hg-HCl to reduce C=O group into >CH2 or use thioacetal and H2/Ni method to do so.
Wait..wrong conversion. Doing it again.
Here :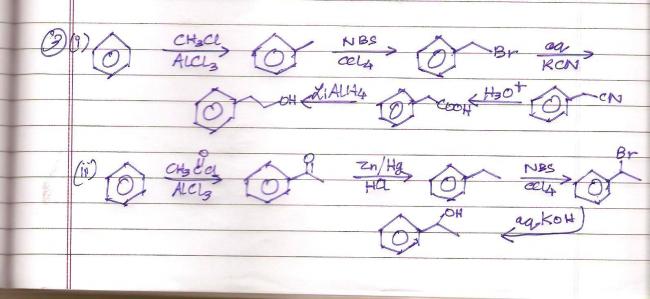
Yeah I think its right..looks more convenient too..board style kar karke kuch zyaada hi lambe ho jaate hai conversions lol
Didn't understand what you said msp...?
Q5 mein LiAlH4 works fine. Had it been an acid derivative I would have preferred DIBAL-H or some sterically hindered reagent.
kk thx...
btw msp i too couldnt understand ur point ...[2]
plz everyone try Q2,4 too
we know dat HF is a weak acid and in the presence of another weak acid its ionisation is further depressed na,so we need energy to dissociate HF where's the energy comes from.