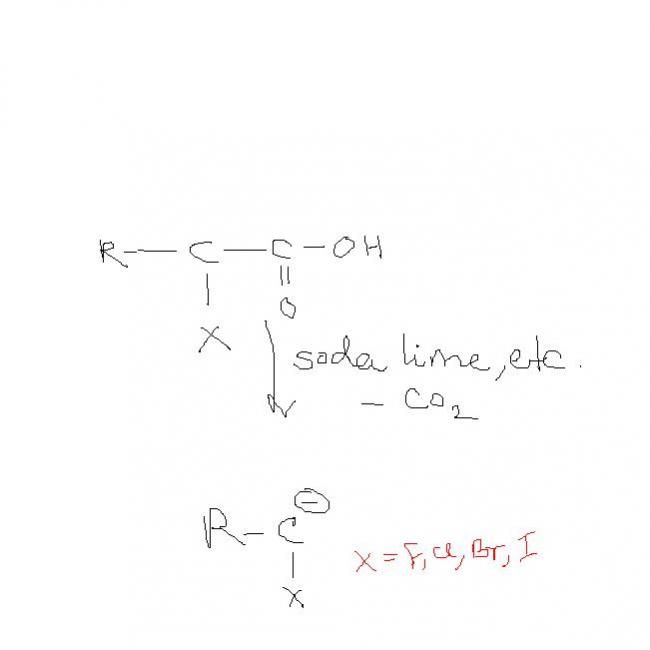
but d ans is ist
compare d rate of decarboxylation
RC(CI)COOH N RC(F)COOH
-
UP 0 DOWN 0 0 24

24 Answers
decarboxylation can occur in the following class of compounds:
(1)β-keto esters
(2)1,1 dicarboxylic acids( generally called "LAMNAC" rule)
(3)β,γ unsaturated acids
additional note: decarbonylation (-CO) occurs in the case of α-keto esters or α-keto acids.
there is no resonace,,,, only inductive effect and we all know -I effect of F is max .......so 2nd carbanion max stable.........so 2nd more reactive
Which type of decarboxylation: Soda lime or the other one (involving electrolysis....i don't remember it's name)?
Cos the latter occurs via free radicle formation.....whose stability can differ with the halogen group attached.
dont say this mathie..................[2]
yesterday only u were solving chem problems in such a gr8888 way......[5]
rate in second will be more...
since electronegativity of F is more than Cl.
so the carbanion formed is more stabilised in second one...
how charge will be more dispersed if F is more Electronegative????
charge will get concentrated about F....[1]
now come on, how come do u say charge is dispersed? R ke place main kuch bhi ho sakhta hain na
ur ans is right but not explaination if f is more electronegative than charge is more dispersed n thus more stable
if thats the case eureka, then obviously first one will b e more stable as chlorine is less elctronegative than flourine.