[1][1][11][12][12][12]
21 Answers
go to ACD labs da . Chemsketch . you have lots of math stuff isko bhi rakh lo . :) very usefu
What is the big deal? Just pass PPL and wait for the results.........
Btw, what sets apart chiral compounds from ordinary compounds, except chiral centres and ability to rotate PPL?
btw with this cyclic chirality there are a lot of issues.. (It is first not in syllabus.. )
so for the ones giving JEE this year should not be reading this.
I cannot draw 3D that easily.. so am having trouble trying to explain the troubles here in this kind of structure..
no dude.. i am not even half learned at this one :)
just trying to help "preeti" who had some 8-10 doubts.. and now i find myself here ;)
NIshant bhay is suprinngly spunning,rocking in org. che as i saw his one more post in some resonance ques. maybe the high scorer of org. in his arena
@nishant
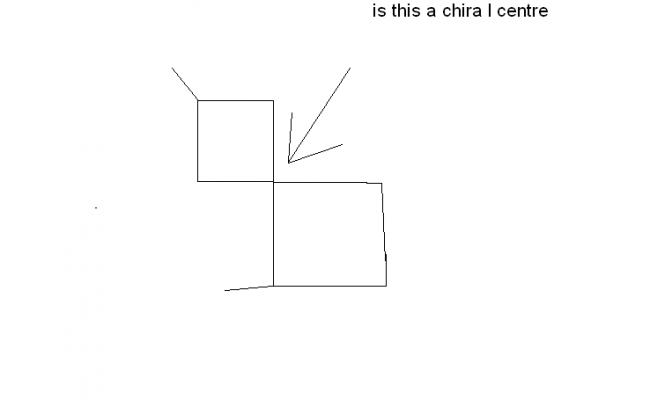
byah, if i place this compound(slightly changed)
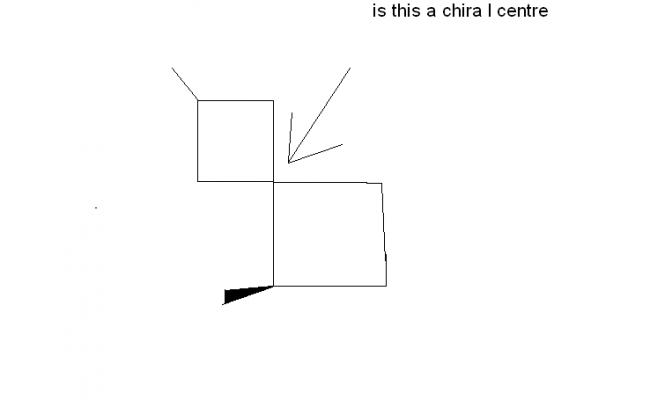
what will u say
and also asish says(3rd post) that the denoted carbon is achiral if things are arrandged symmetrically around it.
which is not the case here..
plz comment
@ asish yeah its op tandon 's GRB,i also feel that many ques . speaciaaly in org. section there are some blunders
@ abhishek..
The central carbon is achiral
but the other carbon which is with the methyl attached to it is chiral. Because it has unsymmetric things around it..
CH3, H and 2 different carbon chains..
OOPS sorry I was seeing a different question.. I will post that :D
i think its no. is it correct? confirm then i will tell you my explanation
nishant bhaiyya, if we see the cyclic ring in which the methyl group is present diagonally opposite to the carbon atom being considered, then whichever way we go, the two groups will be same in all respects. so we cant decide the priority and hence R-S cannot be applied . so it will be achiral as both the groups will be identical.
in a ring to check chirality,if on moving same distances from the carbon to be tested we get identical groups then that carbon is achiral.......[1]
does it not depend on the methyl group there
If the both methyl groups have the same orientation...
I think yes and no depending on how the methyl groups are placed on the ring..!
The problem is actually conceptual :iwant to know how to see chiral when the carbon is a part of a cyclic ring
then we shud wait till others answer. i feel GRB's wrong. btw that's OP Tandon GRB right????
according to me, the carbon u are referring to has two identical groups attached to it.... the one above it with the methyl group attached diagonally, hence it is achiral