I think its mono chloro methane...
Which of the two has greater dipole moment -
A) mono chloro methane 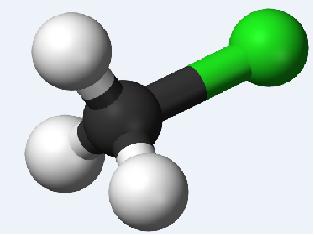
B) tri chloro methane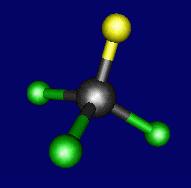
-
UP 0 DOWN 0 0 6

6 Answers
Aditya Bhutra
·2011-06-27 07:24:04
yes it is mono chloro methane but try proving that.
even i was confused at the first go !
Debosmit Majumder
·2011-06-27 10:09:35
i`m sry....a big mistake i made....forgot to consider the angle....the lp-lp repulsion between the chlorine atomes will increase the bond angle from 109.5 to a greater angle and so cos of that angle will be more negative....am i correct? @Aditya....so (a) will be the answer....
Manish Shankar
·2011-06-27 23:49:46
because of repulsion between 3 C-Cl bonds, the three will try to go away towards planarity.
So the net resultant decreases