arey i was wrong ... was drunk :P
jus chcek this link:
http://targetiit.com/iit_jee_forum/posts/dipole_moment_1960.html
this was once discussed in the forum ...
i think CHCl3.
dun ask y .... :P
sky y do u think CHCl3.if we draw
tetrahedral structure of CH2Cl2 AND CHCl3 CLEARLY IT SEEMS THAT CH2Cl2 WILL HAVE MORE DIPOLE MOMENT.....DON U THINK SO!!!!!!!!!!!!!
OKAY.............I M POSTING A REAL TETRAHEDRAL STRUCTURE TO CLEAR MY POINT
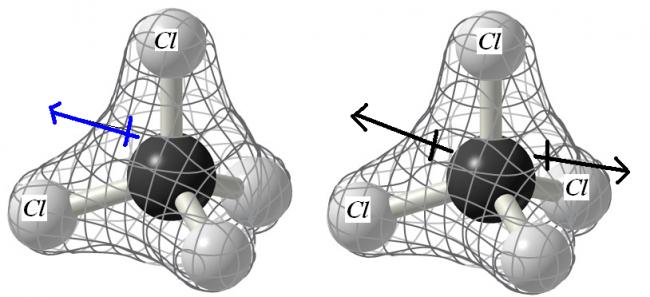
arey i was wrong ... was drunk :P
jus chcek this link:
http://targetiit.com/iit_jee_forum/posts/dipole_moment_1960.html
arey y are u so impatient tell me naa ???
dhoondne me time lagta hai naa....
not being rude... but u c ... [3]
ok chill ! ~
u CHCEK THE LINK!
THE WHOLE LOT OF EXPLANATION EVER REQUIRED TO SOLVE THIS QUESTION IS GIVEN THERE...