now..for that I have no doubt..i agree with others..its HIGH time that they put a RE-TEST..
in the BrF5 question
the asked the number of F-Br-F bonds having 90 degree angle
many say 0 many say 8
but i think answer must be 4
because of the lone pair distortion the four F in the corners of square
are going to be elevated equally annd hence the angle betwwen them
must be 90
so answer must be 4
here is a screen shot of 3d generator of BrF5 molecule
this picture has been taken by keeping the lone pair in front of our eyes
just as we do while assigning R-S notation for enentiomers
plz see
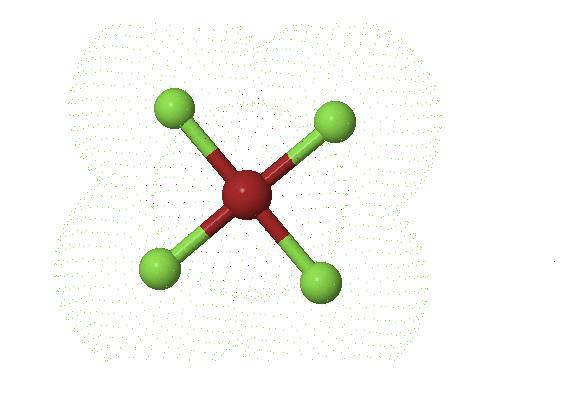
we can clearly see that 4 F-Br-F bonds are 90
-
UP 0 DOWN 0 0 6

6 Answers
ya but this is wat fiitjee says." All four planar bonds (F−Br−F) will reduce from 90° to 84.8° after lp − bp repulsion."
but even thgh i thnk the arrangement wuld have been as lp on the axis with one F and 4 F on the plane, to avoid repulsion so why again we need to consider replulsion for bond angle.
Moreover in the xam i confidently rote the answer as 4 as i thgt i am correct but since then i got to learn solutions of many institutes and every one says it shuld be zero.
I just dont get it how ...[7]
Moreover i have another doubt
why shuld the fission reaction be like this :
92U235 + 0n1 → 54Xe + 38Sr90 + 40n1.
since we know that fission reaction occurs by bombardment of neutrons to the nucleus then how everyone's mentioning reaction as 92U235 → 54Xe + 38Sr90 + 30n1. [7]
i thnk my understanding is reasonable enough.
but IIT this time sucked.[59]
When, i initially saw the prob even i thought the as would be 4.
but the ans is 0 itself.
Take 4 pens in ur hand then tilt all pens below by the same angle 45° just think that would it remain 90°.
no all the 8 angles will change. i dont know by how much exactly but they will surely do change.
try evrything practically u will surely get the ans
for small angles it mey appear that the angle remains 90° but it actually changes although by a small amount