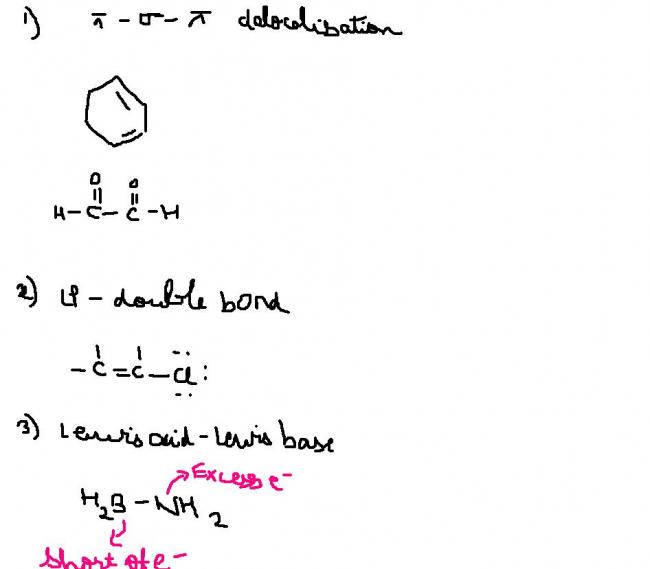by resonance u will get a resonance hybrid for the molecule which shows u the delocalised electron.
explain aromaticity of furan...
There is no delocalisation....
-
UP 0 DOWN 0 0 10

10 Answers
Y THERE IS NO DELOCALIZATION....REMEMBER LONE PAIR ON O AND CONJUGATION!!!![1]
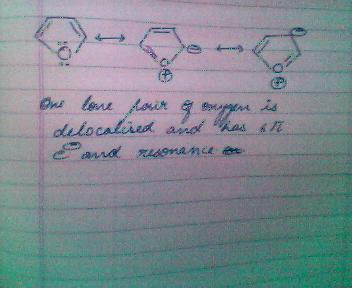
hence one lone pair of the oxygen atom is delocalised which means that this structure is planar, conjugated and obey huckle's rule!!!!!!
hence it is aromatic!!!!!!
@sriraghav ,,, for post#5
aromaticity provides immense stablity ..so if there is any kind of conjugation possible it is bound to happen becoz it would eventually lower the potential energy and lead to stablity of system.
So in this case the lone pair on oxygen atom get delocalised due to conjugation and hence provide stablity.
k ,,,,, thanx a lot....
What i previously thought was there has to be some positive or negative charge on C atom which makes the pi electrons to move around the ring!!
@ sri there are 3 kinds of delocalization posssible..
wait a sec i wiill draw them..