sir can u give me the reason sir why u agreed the srinaths soln.and wats rong with sky sir.
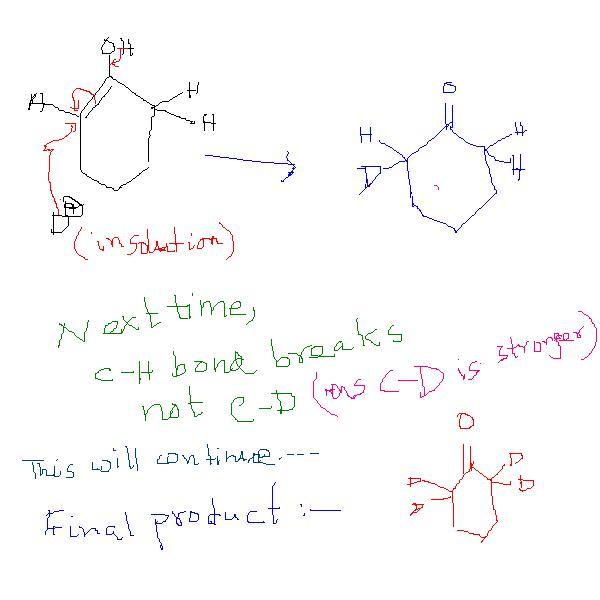
Cyclohexanone is taken in D2O containing OD- and resulting mixture is stirred for several hours to form mainly a compound A.wat is A?
wont it may be a rxn like keto enol conversion.if so please clear.
-
UP 0 DOWN 0 0 22

22 Answers
wat is happening ?
none of the images are getting replaced....
i got the mechanism !!
but [2] .. .... my post ... i canr see..
can nebody see ?
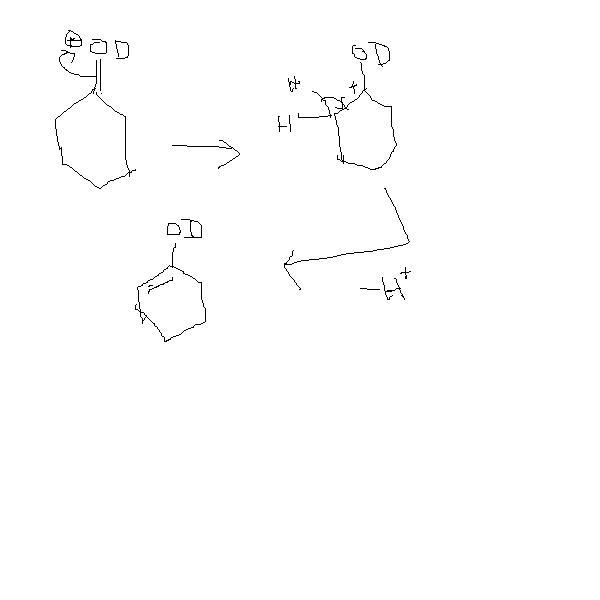
dude. I didn't get by what you meant by this , keto-enol tautomerism can also occur in this condition da. I mean there doesn't seem to be a stabilizing factor for the enol formed.
or may be I'm not understanding what u meant .
please give diag.
sir keto enol tautomerism can also occur under this condition and so the product given by sky may also be correct.
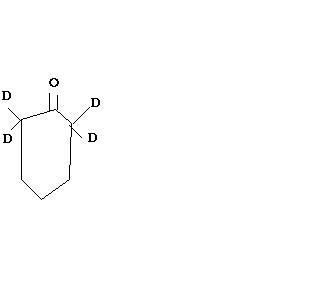
in the first look it seems ok but it is not resulting in product formation and in case of basic medium the alpha H got abstracted by the base and as suggested by srinath the C-D bond is stronger than C-H bond both thermodynamically and kinematically in the final product all alpha H will got replaced by D
why it shd acts as a base,and why it is not undergoing mechanism given by sky.
OD- acts as a base. and then the resulting carbanion will accept a deutron.( deuterium) that's all , the importat part which suggests that all H are replaced is that it's stirred for several hours. as to why CD bond doesn't break is bcoz of the bind strenth . CD > CH.
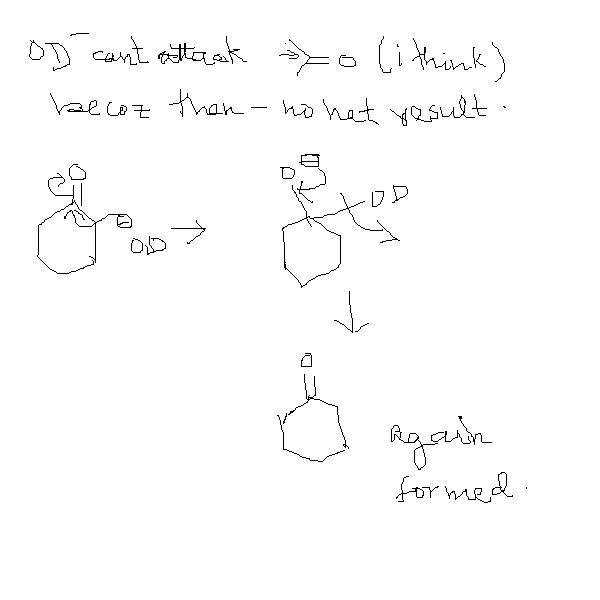
sky u said that OD can't attack , i actuaaly oppose this fact as u see the attach of OD will result in
an intermediate which will form a compound when thwe medium is changed take for e.g. in kolbe's process and many more