1) double bond in between two cyclohexanes with one of the OH remaining.
1.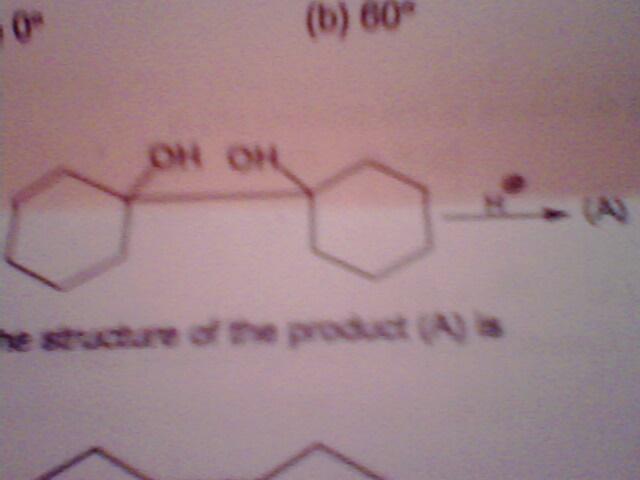
the product formed is?
2.CHBr3+(CH3)3C-OK+cyclohex-1-ene
find the product formed.
3.cyclohexyl chloride is hydrolysed to hydroxycyclohexane slowly, but the reaction fastens when KI is added,because
a.ionic compds.accelerate the reaction
b.in the presence of K+I- ,Cl- becomes a better leaving group.
c.cyclohexyl chloride is unstable in the presence of KI
d.the cyclohexyl iodide formed with KI is more reactive than the corresponding cyclohexyl chloride.
-
UP 0 DOWN 0 0 5

5 Answers
john,sky......bot of you r wrong,options are
1.double bod formed btwn 2 cyclohexanes.
2.the 2nd cyclohexane bcomes 7membered ring with 1 C-O double bond present in the ring.one carbon atom bcomes commn to this ring & the 1st cyclohexane
3.the 2nd cyc.hexane bcoms 5-membered with one C-O double bond &1 carbon atom is commn to this ring&1st cyc. hex.
4.both the rings bcoms 5 membered,are connected to each other by single bond with 1 OH group present on each of these rings