Bisphenol A
http://en.wikipedia.org/wiki/Bisphenol_A
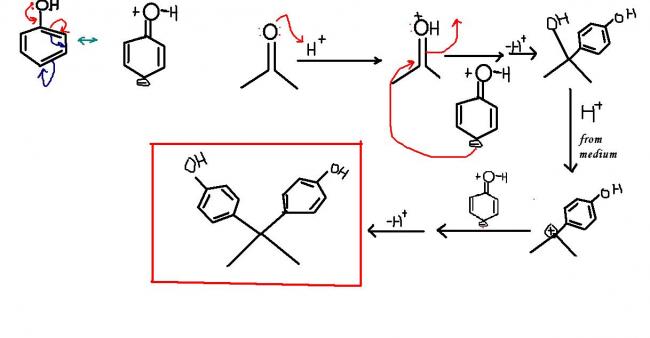
Phenol reacts with acetone in the presence of hydrochloric acid.
Predict the product......Is diphenyl derivative obtained?
Full paper
The reaction yields a product mixture containing bisphenol A, and also unreacted phenol, unreacted acetone, hydrochloric acid, water, and by-products. The product mixture is distilled under reduced pressure to remove water, acetone, hydrochloric acid, and a small amount of phenol. The vacuum distillation should be performed preferably at a pressure of 20 to 200 mmHg and a temperature of 90 to 100° C. Thus there is obtained a phenol solution of crude bisphenol A.
The thus-obtained phenol solution of crude bisphenol A is cooled to 35 to 70° C. in a crystallizer so that the adduct of bisphenol A with phenol crystallizes. The crystallization is accomplished by adding water to the crystallizer and evaporating water and phenol under reduced pressure (preferably 20 to 100 mmHg), thereby removing heat. The evaporation yields a distillate composed mainly of a mixture of water and a small amount of phenol. This mixture is treated with a weakly basic ion-exchange resin, and the treated mixture is recycled and reused as the water to be added to the crystallizer.
The amount of the water-phenol mixture to be recycled should be sufficient to cool the phenol solution of crude bisphenol A and also to remove the heat of crystallization of the adduct by its evaporation. This amount is 2 to 20 wt% of the phenol solution.
The weakly basic ion-exchange resins used in-the process of the present invention should preferably be substantially insoluble in the water-phenol mixture. They include ion-exchange resins which contain secondary or tertiary amine as the exchange group, for example, "LEWATIT MP-62" (Bayer AG) and "WA-20" (Mitsubishi Chemical Industries, Ltd.). The treatment with the ion-exchange resin should preferably be carried out continuously at 20 to 70° C. The amount of the water-phenol mixture to be added to the ion-exchange resin should preferably be 0.5 to 10 kg/hr for 1 kg of the ion-exchange resin.
According to the process of the present invention, the crystals of the adduct of bisphenol A with phenol are separated by any known method, and the bisphenol A is freed of phenol.
------Iimuro, Shigeru (Aichi, JP)
Morimoto, Yoshio (Aichi, JP)
Kitamura, Takashi (Aichi, JP)
.....NB: we follow the same mechanism in preparing phenolphthalein from thallic anhydride