yup...ashish is correct ! [1]
ARRANGE THE FOLLOWING IN DECREASING ORDER OF BASICITY. eXPLAIN THE REASON AS WELL.
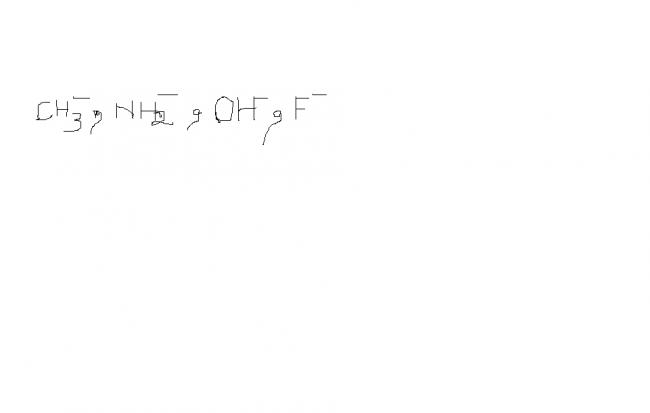
-
UP 0 DOWN 0 0 2

2 Answers
Asish Mahapatra
·2009-12-10 22:50:56
See,
HF is most acidic followed by H2O then NH3 and last CH4 ( i think u can reason this out)
now their conjugate bases are
F- , OH- ,, NH2- ,, CH3-
now stronger acids have weaker conjugate bases,
so strongest base is the one corresponding to weakest acid
so, it is
CH3- > NH2- > OH- > F-