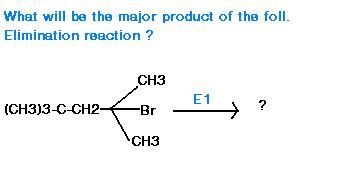
Hoffmann elimination occurs in substrates containing alkyl trimethyl amines or the like. Here it will be E1 only...or so I think.
34 Answers
oops....sorry.. i meant to say... that in E1, Saytzeff's rule is used and the most substituted alkene is formed. In this case it would be the one i've shown above.
Actually, in E2 mechanism also, Saytzeff's rule is applicable.
Last Option left fr us ~ The answer is wrong....Is it ?
(Actually it was a MCQ single answer ,n our Saytzeff product answer was also as one of the options...so, a damn mistake again, i presume !?)
For those thinking Hoffman Elimination is possible, I picked this up from Wiki -:
The Zaitsev rule is correct only when there are no other substituents beside carbon and hydrogen. Once other atoms are added; electronegativity, resonance, and other factors complicate the situation and invalidate the rule.